When zinc is added to a blue solution of copper sulfate, a chemical reaction occurs. Copper settles from the solution as an orange-brown precipitate, zinc dissolves into the solution, and the blue colour of the solution fades. The mass of copper formed is approximately equal to the mass of zinc that dissolves. A student predicted that 1 kg of solid copper sulfate should contain 250 g of pure copper. To test the prediction, a 10.0 g sample of blue copper sulfate crystals was dissolved in water. 5.0 g of zinc metal were added to the solution. The solution was left to stand until the blue colour had completely disappeared. The resulting solid was separated, dried thoroughly and weighed. Its mass was 4.9 g. Which statement is correct? А. The student's prediction is supported because the mass measured was very close to the expected result. В. The student's prediction is not supported. The mass measured is too large because not all the copper sulfate has reacted. C. The student's prediction is not supported. The mass measured is too small because insufficient zinc was added to the solution. D. The student's prediction is not supported. The mass measured was too large because there is unreacted zinc mixed with the copper.
When zinc is added to a blue solution of copper sulfate, a chemical reaction occurs. Copper settles from the solution as an orange-brown precipitate, zinc dissolves into the solution, and the blue colour of the solution fades. The mass of copper formed is approximately equal to the mass of zinc that dissolves. A student predicted that 1 kg of solid copper sulfate should contain 250 g of pure copper. To test the prediction, a 10.0 g sample of blue copper sulfate crystals was dissolved in water. 5.0 g of zinc metal were added to the solution. The solution was left to stand until the blue colour had completely disappeared. The resulting solid was separated, dried thoroughly and weighed. Its mass was 4.9 g. Which statement is correct? А. The student's prediction is supported because the mass measured was very close to the expected result. В. The student's prediction is not supported. The mass measured is too large because not all the copper sulfate has reacted. C. The student's prediction is not supported. The mass measured is too small because insufficient zinc was added to the solution. D. The student's prediction is not supported. The mass measured was too large because there is unreacted zinc mixed with the copper.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter2: Chemical Compounds
Section: Chapter Questions
Problem 105QRT
Related questions
Question
pls assist. thanks
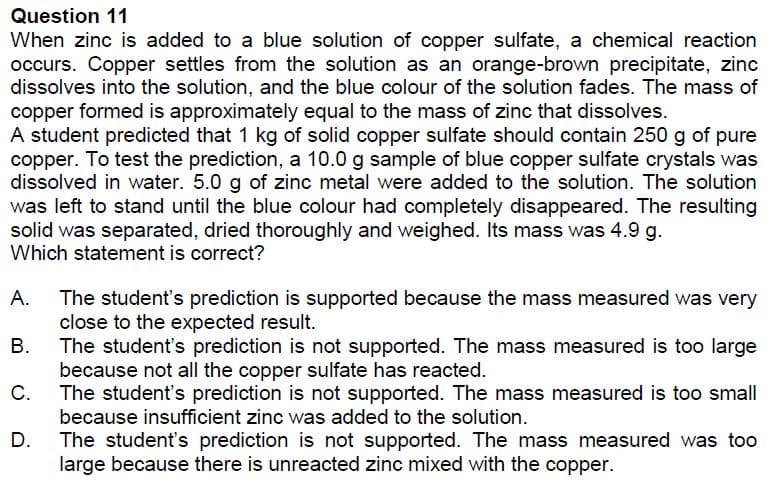
Transcribed Image Text:Question 11
When zinc is added to a blue solution of copper sulfate, a chemical reaction
occurs. Copper settles from the solution as an orange-brown precipitate, zinc
dissolves into the solution, and the blue colour of the solution fades. The mass of
copper formed is approximately equal to the mass of zinc that dissolves.
A student predicted that 1 kg of solid copper sulfate should contain 250 g of pure
copper. To test the prediction, a 10.0 g sample of blue copper sulfate crystals was
dissolved in water. 5.0 g of zinc metal were added to the solution. The solution
was left to stand until the blue colour had completely disappeared. The resulting
solid was separated, dried thoroughly and weighed. Its mass was 4.9 g.
Which statement is correct?
А.
The student's prediction is supported because the mass measured was very
close to the expected result.
В.
The student's prediction is not supported. The mass measured is too large
because not all the copper sulfate has reacted.
C.
The student's prediction is not supported. The mass measured is too small
because insufficient zinc was added to the solution.
D.
The student's prediction is not supported. The mass measured was too
large because there is unreacted zinc mixed with the copper.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning