Which equation shows a weak acid in water? O LIOH(s) –-> Li* (aq) + OH(aq) O HF(aq) + H,0(1) <→ H30*(aq) + F(aq) O HI(aq) eOH* (aq) + |(aq) O HCl(aq) + H2O(aq) --> CI (aq) + H;O*(aq)
Which equation shows a weak acid in water? O LIOH(s) –-> Li* (aq) + OH(aq) O HF(aq) + H,0(1) <→ H30*(aq) + F(aq) O HI(aq) eOH* (aq) + |(aq) O HCl(aq) + H2O(aq) --> CI (aq) + H;O*(aq)
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter16: Principles Of Chemical Reactivity: The Chemistry Of Acids And Bases
Section: Chapter Questions
Problem 7PS: In each of the following acid-base reactions, identify the Brnsted acid and base on the left and...
Related questions
Question
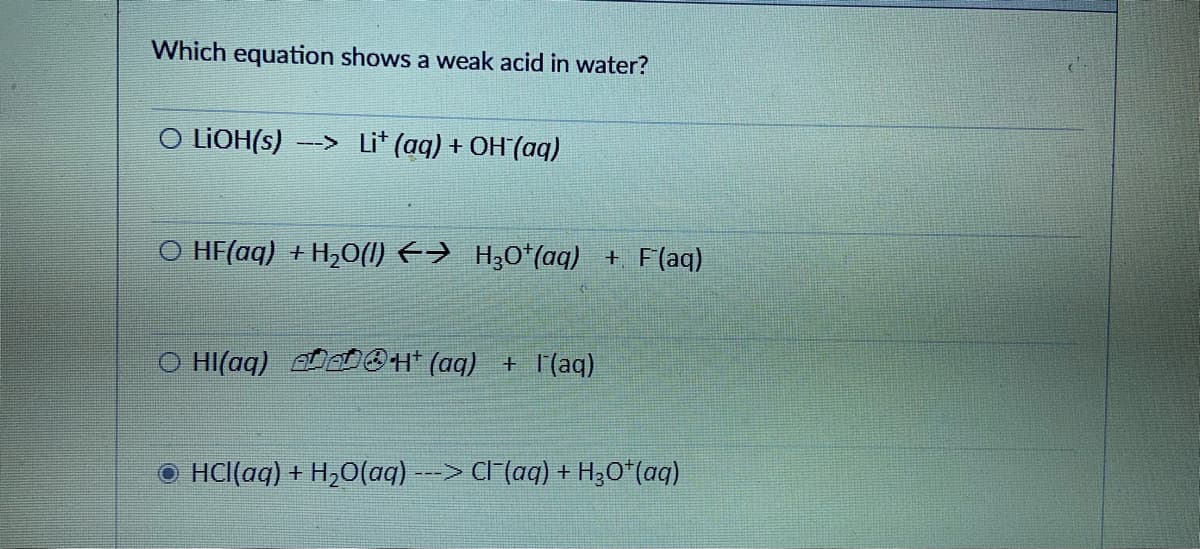
Transcribed Image Text:Which equation shows a weak acid in water?
O LIOH(s)
--> Li* (aq) + OH (aq)
O HF(aq) + H,0(1) E→ H30*(aq) + F(aq)
O HI(aq)
OH (aq) + l'(aq)
O HCl(aq) + H,O(aq) ---> Cl (aq) + H;O*(aq)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
