Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter4: Chemical Foundations: Elements, Atoms, And Ions
Section: Chapter Questions
Problem 91AP: Is it possible for the same Iwo elements to form more than one compound? Is this consistent with...
Related questions
Question
100%
Transcribed Image Text:AMoving to another question will save this response.
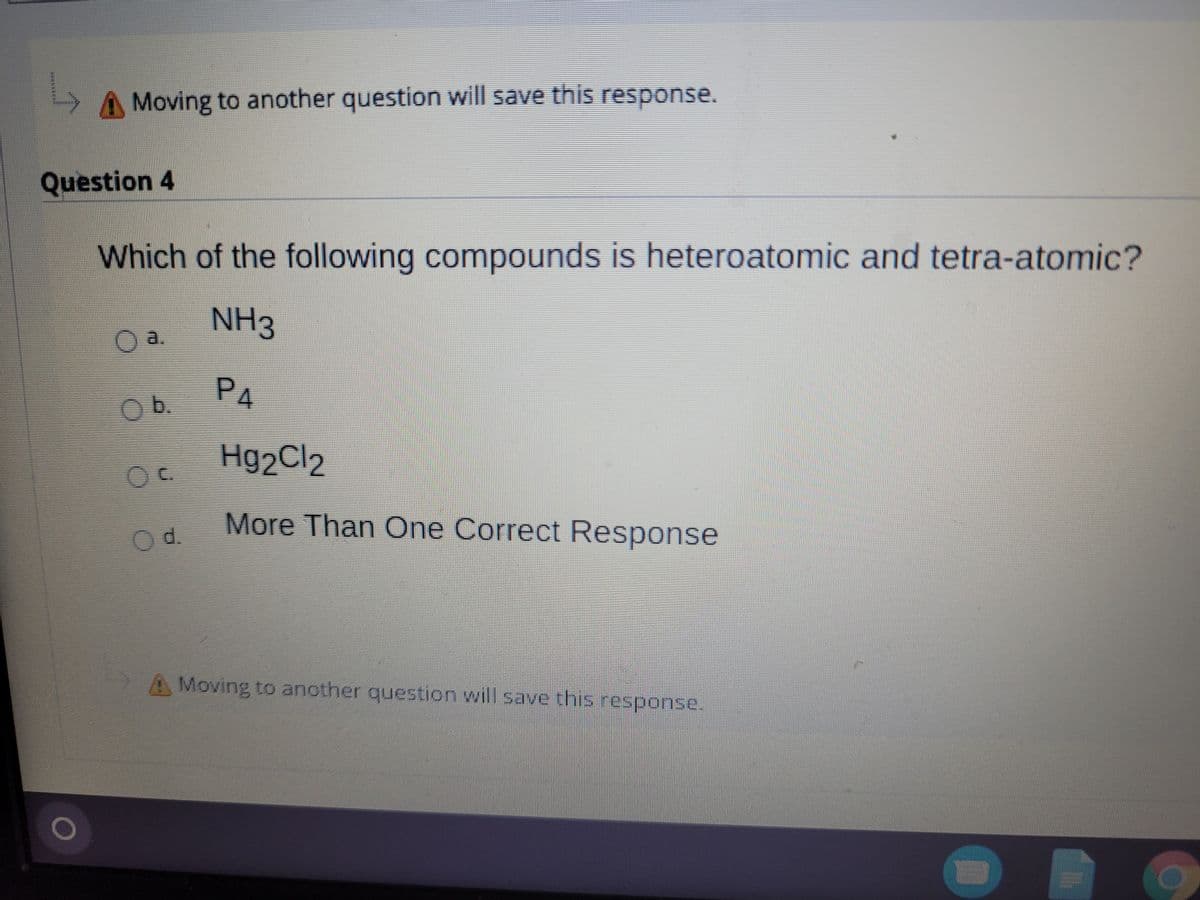
Question 4
Which of the following compounds is heteroatomic and tetra-atomic?
NH3
Oa.
PA
Ob.
Hg2Cl2
More Than One Correct Response
Od.
A Moving to another question will save this response.
Expert Solution
Step 1
Heteroatomic compound :-
A compound in which its molecules consists of more than one type of atoms is called heteroatomic compound .
For example :-
H2O is heteroatomic , as it consists of two different atoms H and O
Homoatomic compound :-
A compound in which its molecules consists of only one type of atoms is called homoatomic compound .
For example :-
H2 is homoatomic , as it consists of only one type of atoms
Atomicity :-
The number of atoms present in a molecule of a compound is called atomicity .
H2O is tri-atomic as it consists of three atoms
H2O2 is tetra-atomic as consists of four atoms.
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning