Which of the following has the greatest solubility in CH3CH2CH,CH2CH2CH3? Select one: O a. CH3OH O b. CH3NH2 O c. CH3OCH3 O d. CH3O Na* O e. (CH3)3CH Clear my choice Which of the following correctly ranks the cycloalkanes in order of increasing ring strain? Select one: O a. cyclopentane < cyclopropane < cyclobutane < cyclohexane O b. cyclohexane < cyclopentane < cyclobutane < cyclopropane O c. cyclopropane < cyclobutane < cyclohexane < cycloheptane O d. cyclopropane < cyclopentane < cyclobutane < cyclohexane O e. cyclopentane < cyclobutane < cyclopentane < cyclopropane Clear my choice
Which of the following has the greatest solubility in CH3CH2CH,CH2CH2CH3? Select one: O a. CH3OH O b. CH3NH2 O c. CH3OCH3 O d. CH3O Na* O e. (CH3)3CH Clear my choice Which of the following correctly ranks the cycloalkanes in order of increasing ring strain? Select one: O a. cyclopentane < cyclopropane < cyclobutane < cyclohexane O b. cyclohexane < cyclopentane < cyclobutane < cyclopropane O c. cyclopropane < cyclobutane < cyclohexane < cycloheptane O d. cyclopropane < cyclopentane < cyclobutane < cyclohexane O e. cyclopentane < cyclobutane < cyclopentane < cyclopropane Clear my choice
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter2: Alkanes And Cycloalkanes
Section: Chapter Questions
Problem 2.45P: Gibbs free energy differences between axial-substituted and equatorial-substituted chair...
Related questions
Question
Are my answers correct?
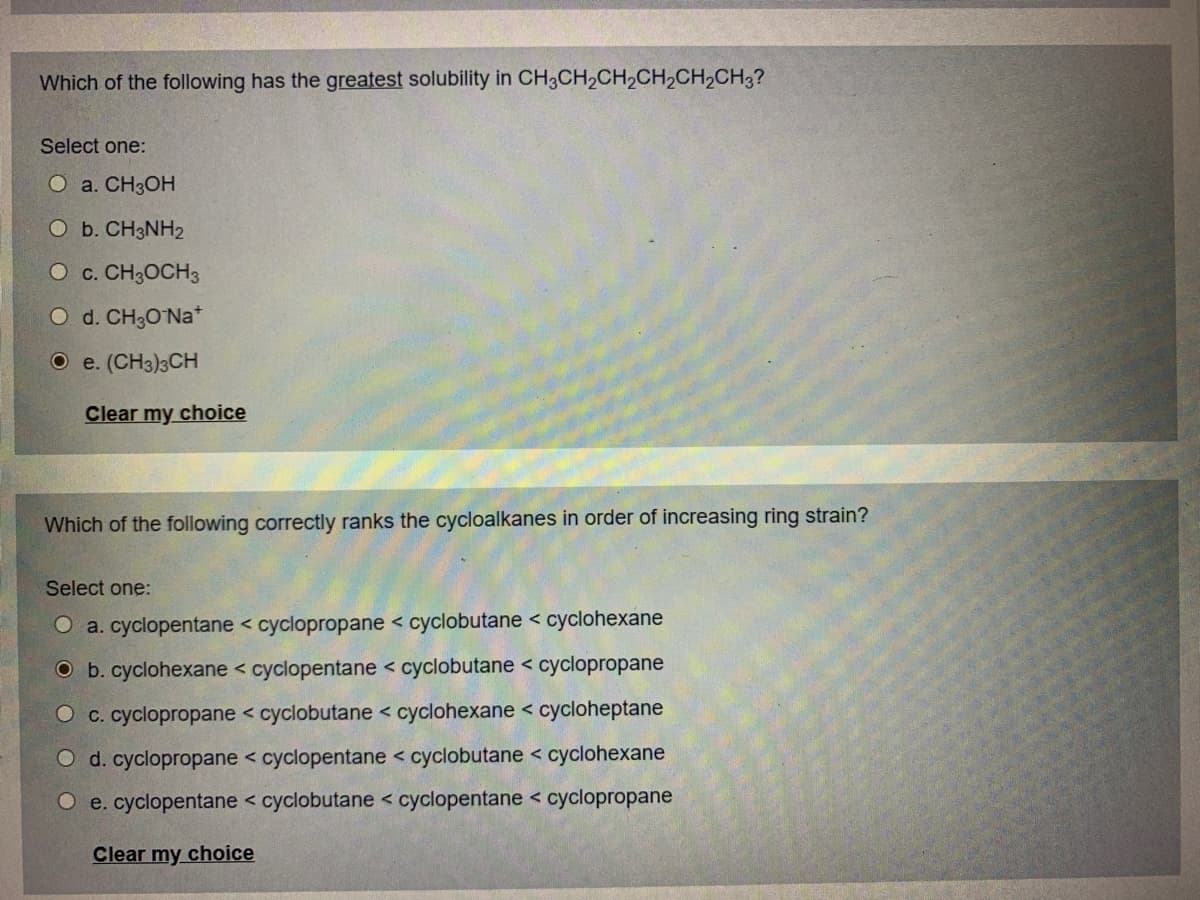
Transcribed Image Text:Which of the following has the greatest solubility in CH3CH2CH,CH2CH2CH3?
Select one:
O a. CH3OH
O b. CH3NH2
O c. CH3OCH3
O d. CH3O Na*
O e. (CH3)3CH
Clear my choice
Which of the following correctly ranks the cycloalkanes in order of increasing ring strain?
Select one:
O a. cyclopentane < cyclopropane < cyclobutane < cyclohexane
O b. cyclohexane < cyclopentane < cyclobutane < cyclopropane
O c. cyclopropane < cyclobutane < cyclohexane < cycloheptane
O d. cyclopropane < cyclopentane < cyclobutane < cyclohexane
O e. cyclopentane < cyclobutane < cyclopentane < cyclopropane
Clear my choice
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning