Which of the following is a correct net ionic equation for the reaction of a metal and an acid? ○ Mg (s) + 2 H+ (aq) → 2 Mg2+ (aq) + H₂ (8) K (s) + HBr (aq) → KH (s) + Br2 (g) Na (s) + 3 H+ (aq) → Na+ + 3 H (g) 2 Al (s) + 3 H2SO4 (aq) → Al2(SO4)3 (aq) + 3 H₂ (g)
Which of the following is a correct net ionic equation for the reaction of a metal and an acid? ○ Mg (s) + 2 H+ (aq) → 2 Mg2+ (aq) + H₂ (8) K (s) + HBr (aq) → KH (s) + Br2 (g) Na (s) + 3 H+ (aq) → Na+ + 3 H (g) 2 Al (s) + 3 H2SO4 (aq) → Al2(SO4)3 (aq) + 3 H₂ (g)
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter3: Chemical Reactions
Section: Chapter Questions
Problem 44QRT
Related questions
Question
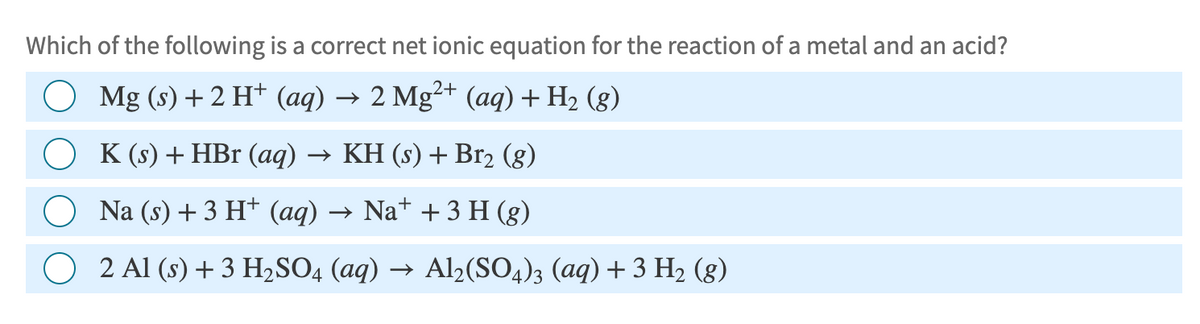
Transcribed Image Text:Which of the following is a correct net ionic equation for the reaction of a metal and an acid?
○ Mg (s) + 2 H+ (aq) → 2 Mg2+ (aq) + H₂ (8)
K (s) + HBr (aq) → KH (s) + Br2 (g)
Na (s) + 3 H+ (aq) → Na+ + 3 H (g)
2 Al (s) + 3 H2SO4 (aq) → Al2(SO4)3 (aq) + 3 H₂ (g)
AI-Generated Solution
Unlock instant AI solutions
Tap the button
to generate a solution
Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning