Q: Write the following equation with correct formulas, balance, and describe the type of reaction it…
A: Introduction - Single replacement reaction: A + BC →B + AC. This type of one element replacement…
Q: The drawing below shows a mixture of molecules:
A:
Q: Why do decomposition reactions typically have compoundsas reactants, whereas combination and…
A: Given reactions, Decomposition reaction, Combination and displacement reaction.
Q: Which type will have two elements as a reactants.
A: Synthesis reaction will have two different elements or molecules as reactants that interact to form…
Q: Which of the following is a precipitation reaction?
A: 2 option is correct.
Q: Classify the following as combination, decomposition, substitution, or double displacement…
A: The chemical reactions may takes place by combination or decomposition or displacement. The types of…
Q: In the balanced molecular equation for the neutralization of sodium hydroxide with sulfuric acid,…
A: The balanced molecular equation for the neutralization of sodium hydroxide with sulfuric acid is…
Q: A precipitate is formed when
A: Precipitate is an insoluble solid mass that is formed by precipitation reaction.
Q: What is the difference between the following symbols in chemical equations: → and ⇌?(
A: A chemical reaction is the representation of the conversion of the reactants into products.…
Q: a precipitate when mixed?
A: From the given pairs of reagents, which will form precipitate , when they were mixed has to be…
Q: What molecules can be made if the ions react with water?
A:
Q: Describe an example of novel reactivity that occurs when using ionic liquids as solvents.
A:
Q: If no precipitate is observed when hydrochloric acid is added to a solution that contains a mixture…
A: A precipitate is formed when HCl is added to a solution containing mixture of cations when it forms…
Q: Direction : Write the reactions given in table 1,that corresponds to the given evidences below :…
A: A question based on chemical reaction and evidences, which is to be accomplished.
Q: Which of the following is an acid base reaction?
A:
Q: According to the solubility rules, which one of the following should be soluble in water?
A: Which silver salt would be soluble in water.
Q: Write a chemical equation for solid cadmium hydrogen carbonate decomposing to yield solid cadmium…
A: The chemical equation for solid cadmium hydrogen carbonate decomposing to yield solid cadmium…
Q: If aqueous solutions of sodium bromide and calcium nitrate and are mixed, will a precipitate form?…
A: Precipitation Reactions are those reactions in which the cations and anions in aqueous solution…
Q: In the following equation, __________ will form a precipitate.
A: The branch of chemistry that deals with the structure, properties and the reaction of the organic…
Q: Which of the following reactants is a base?
A: When an acid reacts with a base it produces salt and water. Such reactions are known as acid-base…
Q: Write balanced chemical equations for each of the acid-base reactions described below.
A: Balance chemical equation is defined as which has equal number of atoms or ions in both sides…
Q: How many grams of Cu(OH)2 will precipitate
A:
Q: What are two products when an acid and base react?
A: Given Reactant = Acid ,Base Product = ?
Q: What ions exist in acid and base solutions?
A: According to Arrhenius, Acids are those which produces H+ ions in the solution Bases are those which…
Q: solubility of oz
A:
Q: Two solutions, when mixed together at room temperature, producea chemical reaction. However, when…
A: When two or more substances combine resulting in the formation of a new substance, a chemical…
Q: What are, are complete ionic equations?
A: Based on representation of reactants and product as ions or molecules in aqueous solution, we can…
Q: Which ion(s) is/are spectator ions in the formation of a precipitate when combining aqueous…
A: Spectator ions are those which remains unchanged (Concentration of ions) after the completion of…
Q: What concentration of sulfuric acid remains
A:
Q: The net ionic equation for the reaction of aqueous lead (II) nitrate and aqueous ammonium phosphate…
A: The given reaction is an example of double displacement reaction. In this reaction, the cationic and…
Q: Solid sodium bicarbonate reacted with aqueous Hydrochloric acid(hydrogen monochloride) to form…
A:
Q: Write a balanced chemical equation for reaction. Aqueous hydrochloric acid (HCl) reacts with solid…
A: Balanced chemical equation: It is form of writing chemical reaction in which all elements present at…
Q: Which of the following provides the best evidence that a chemical reaction has taken place? A Water,…
A: D. Two liquids are mixed, and a solid substance forms. This evidence provides the best evidence…
Q: Which of the following compound CAN FORM precipitate? A. Calcium chloride B. Silver nitrate C.…
A: Most of the nitrate salts are soluble.Chlorides of Ag+, Pb+2 are not soluble carbonates of alkali…
Q: Write an equation for the precipitation reaction (if any) that occurs when solutions of lead(II)…
A: A reaction is called precipitation reaction when two solution containing soluble salt are combined…
Q: What is the difference between the following symbolsin chemical equations: → and ⇌?
A: The chemical reaction is the reaction in which reactants are combined to form products under…
Q: which of the following reactions shows precipitation reaction?
A: The reaction in which when two salt solutions which are soluble react with each other forms an…
Q: What are three observations that would indicate a chemical reaction has taken place?
A: When a chemical reaction takes place, some changes are observed. These changes help us to check that…
Q: What does the base do in an acid-base reaction?
A: In acid base reaction , when acid contains H+ions react with base contains OH-ions it forms salt and…
Q: Data: Mass of filter paper 0.750 grams Mass of filter paper and precipitate 3.258 grams 2.659 grams…
A:
Q: What precipitate forms when aqueous lead (II) nitrate reacts with aqueous potassium iodide?…
A: This is an example of double displacement reaction.
Q: 4
A: Please find the file attached for explanation
Q: Which of the following reactions are written correctly?
A: Acid : It increase H+ ion concentration in the solution ex: HCl , H2SO4 , HNO3 etc Depending…
Q: Write a balanced chemical equation based on the following descriptio solid magnesium metal reacts…
A: According to law of conservation of mass, in a chemical reaction mass of products and reactants…
Q: Which of the following represents a precipitation reaction?
A: Precipitation reaction: Reaction in which precipitate form (in form of insoluble salt) is called…
Q: What occurs on the molecular level when an ionic compound dissolves in water?
A: Ionic compounds are the neutral compounds, Ions are of two types: cation and anion. Due to loss of…
Q: Nitrogen dioxide dissolves in water to form nitric acid and nitric oxide.
A: A balanced reaction is a reaction where the number of atoms of each element is same on both reactant…
Q: How would an imbalance chemical equation affect a chemical reaction? And how would you determine…
A: An equation must be balanced. If it is not balanced, then it will oppose the law of conservation of…
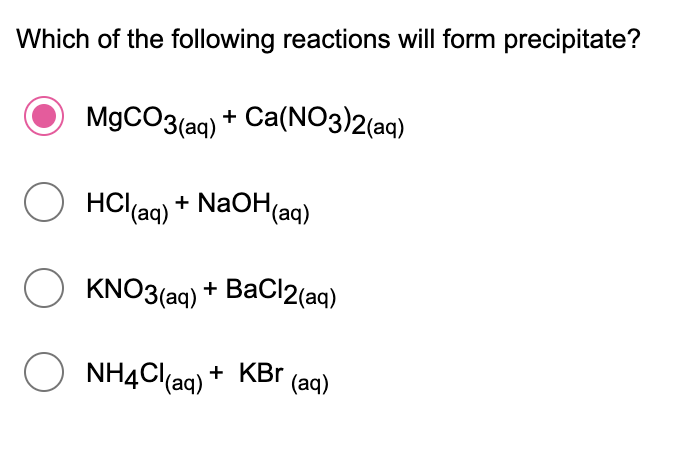
Step by step
Solved in 3 steps

- Consider the following generic equation: H+(aq)+ B(aq)HB(aq)For which of the following pairs would this be the correct prototype equation for the acid-base reaction in solution? If it is not correct, write the proper equation for the acid-base reaction between the pair. (a) nitric acid and calcium hydroxide (b) hydrochloric acid and CH3NH2 (c) hydrobromic acid and aqueous ammonia (d) perchloric acid and barium hydroxide (e) sodium hydroxide and nitrous acidOn Easter Sunday, April 3, 1983, nitric acid spilled from a tank car near downtown Denver, Colorado. The spill was neutralized with sodium carbonate: 2HNO3(aq)+Na2CO3(aq)2NaNO3(aq)+H2O(l)+CO2(g) a. Calculate H for this reaction. Approximately 2.0 104 gal nitric acid was spilled. Assume that the acid was an aqueous solution containing 70.0% HNO3 by mass with a density of 1.42 glcm3. What mass of sodium carbonate was required for complete neutralization of the spill, and what quantity of heat was evolved? (Hf for NaNO3(aq) = 467 kJ/mol) b. According to The Denver Post for April 4, 1983, authorities feared that dangerous air pollution might occur during the neutralization. Considering the magnitude of H, what was their major concern?A noncarbonated soft drink contains an unknown amount of citric acid, H3C6H5O7. lf 100. mL of the soft drink requires 33.51 mL of 0.0102 M NaOH to neutralize the citric add completely, what mass of citric acid does the soft drink contain per 100. mL? The reaction of citric acid and NaOH is H3C6H5O7(aq) + 3 NaOH(aq) Na3C6H5O7(aq) + 3 H2O()
- 1. Sometimes a reaction can fall in more than one category. Into what category (or categories) does the reaction of Ba(OH)2(aq) + H+PO4(aq) fit? acid-base and oxidation-reduction oxidation-reduction acid-base and precipitation precipitationCitric acid, which can be obtained from lemon juice, has the molecular formula C6H8O7. A 0.250-g sample of citric acid dissolved in 25.0 mL of water requires 37.2 mL of 0.105 M NaOH for complete neutralization. What number of acidic hydrogens per molecule does citric acid have?Vitamin C, ascorbic acid (C6HgO6)(molar mass 176.1 g/mol), is a reducing agent. One way to determine the ascorbic acid content of a sample is to mix the acid with an excess of iodine, C6HgO6(aq) + I2(aq) + H2O(l) C6HgO6(aq) + 2 H3O+(aq) + 2 I(aq) and then titrate the iodine that did not react with the ascorbic acid with sodium thiosulfate. The balanced, net ionic equation for the reaction occurring in this titration is I2(aq) + 2 S2O32(aq)2 I(aq) + S4O62(aq) Suppose 50.00 mL of 0.0520 M I2 was added to the sample containing ascorbic acid. After the ascorbic acid/I2, reaction was complete, the I2 not used in this reaction required 20.30 mL of 0.196 M Na2S2O3 for titration to the equivalence point. Calculate the mass of ascorbic acid in the unknown sample.
- Aqueous solutions of ammonium sulfide and mercury(II) nitrate react and a precipitate forms. (a) Write the overall balanced chemical equation and indicate the state (aq) or (s) for each compound. (b) Name each product. (c) Write the complete ionic equation. (d) Write the net ionic equation.Consider the following generic equation OH(aq)+HB(aq) B(aq)+H2OFor which of the following pairs would this be the correct prototype equation for the acid-base reaction in solution? If it is not correct, write the proper equation for the acid-base reaction between the pair. (a) hydrochloric acid and pyridine, C5H5N (b) sulfuric acid and rubidium hydroxide (c) potassium hydroxide and hydrofluoric acid (d) ammonia and hydriodic acid (e) strontium hydroxide and hydrocyanic acidA 300.0-g sample of a solid is made up of a uniform mixture of NaNO3, MgCl2, and BaCl2. A 100.0-g sample of the mixture is dissolved in water and treated with an excess of KOH. The precipitate from the reaction has a mass of 13.47 g. The remaining 200.0-g sample is also dissolved in water and treated with an aqueous solution of AgNO3. The resulting precipitate has a mass of 195.8 g. What are the masses of NaNO3, MgCl2, and BaCl2 in the 300.0-g sample?
- onsider separate aqueous solutions of HCI and H2S04 with the same concentrations in terms of molarity. You wish to neutralize au aqueous solution of’ NaOH. For which acid solution would you need to add more volume (in mL) to neutralize the base? The HCI solution. The H2SO4 solution. You need to know the acid concentrations to answer this question. You need to know the volume and concentration of’ the NaOH solution to answer this question. c and d plain your answer.The Behavior of Substances in Water Part 1: a Ammonia, NH3, is a weak electrolyte. It forms ions in solution by reacting with water molecules to form the ammonium ion and hydroxide ion. Write the balanced chemical reaction for this process, including state symbols. b From everyday experience you are probably aware that table sugar (sucrose), C12H22O11, is soluble in water. When sucrose dissolves in water, it doesnt form ions through any reaction with water. It just dissolves without forming ions, so it is a nonelectrolyte. Write the chemical equation for the dissolving of sucrose in water. c Both NH3 and C12H22O11 are soluble molecular compounds, yet they behave differently in aqueous solution. Briefly explain why one is a weak electrolyte and the other is a nonelectrolyte. d Hydrochloric acid, HCl, is a molecular compound that is a strong electrolyte. Write the chemical reaction of HCl with water. e Compare the ammonia reaction with that of hydrochloric acid. Why are both of these substances considered electrolytes? f Explain why HCl is a strong electrolyte and ammonia is a weak electrolyte. g Classify each of the following substances as either ionic or molecular. KCl NH3 CO2 MgBr2 HCl Ca(OH)2 PbS HC2H3O2 h For those compounds above that you classified as ionic, use the solubility rules to determine which are soluble. i The majority of ionic substances are solids at room temperature. Describe what you would observe if you placed a soluble ionic compound and an insoluble ionic compound in separate beakers of water. j Write the chemical equation(s), including state symbols, for what happens when each soluble ionic compound that you identified above is placed in water. Are these substances reacting with water when they are added to water? k How would you classify the soluble ionic compounds: strong electrolyte, weak electrolyte, or nonelectrolyte? Explain your answer. l Sodium chloride, NaCl, is a strong electrolyte, as is hydroiodic acid, HI. Write the chemical equations for what happens when these substances are added to water. m Are NaCl and HI strong electrolytes because they have similar behavior in aqueous solution? If not, describe, using words and equations, the different chemical process that takes place in each case. Part 2: You have two hypothetical molecular compounds, AX and AY. AX is a strong electrolyte and AY is a weak electrolyte. The compounds undergo the following chemical reactions when added to water. AX(aq)+H2O(l)AH2O+(aq)+X(aq)AY(aq)+H2O(l)AH2O+(aq)+Y(aq) a Explain how the relative amounts of AX(aq) and AY(aq) would compare if you had a beaker of water with AX and a beaker of water with AY. b How would the relative amounts of X(aq) and Y(aq) in the two beakers compare? Be sure to explain your answer.39. Standard solutions of calcium ion used to test for water hardness are prepared by dissolving pure calcium carbonate. CaCO3, in dilute hydrochloric acid. A 1.745-g sample of CaCO3 is placed in a 250.O-mL volumetric flask and dissolved in HCI. Then the solution is diluted to the calibration mark of the volumetric flask. Calculate the resulting molarity of calcium ion.











