Which of the following statements about the Kinetic Theory of gases is false? o Kinetic energy of a gas molecule is directly related to the temperature. O Gases consist of molecules in continuous, random, straight-line motion. O Collisions between molecules are elastic. O Gas particles are much larger than the distances betwveen them. « Previous No new data to save, Last checked at
Which of the following statements about the Kinetic Theory of gases is false? o Kinetic energy of a gas molecule is directly related to the temperature. O Gases consist of molecules in continuous, random, straight-line motion. O Collisions between molecules are elastic. O Gas particles are much larger than the distances betwveen them. « Previous No new data to save, Last checked at
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter13: Gases
Section: Chapter Questions
Problem 4ALQ
Related questions
Question
Help number 3 please
Transcribed Image Text:Question 3
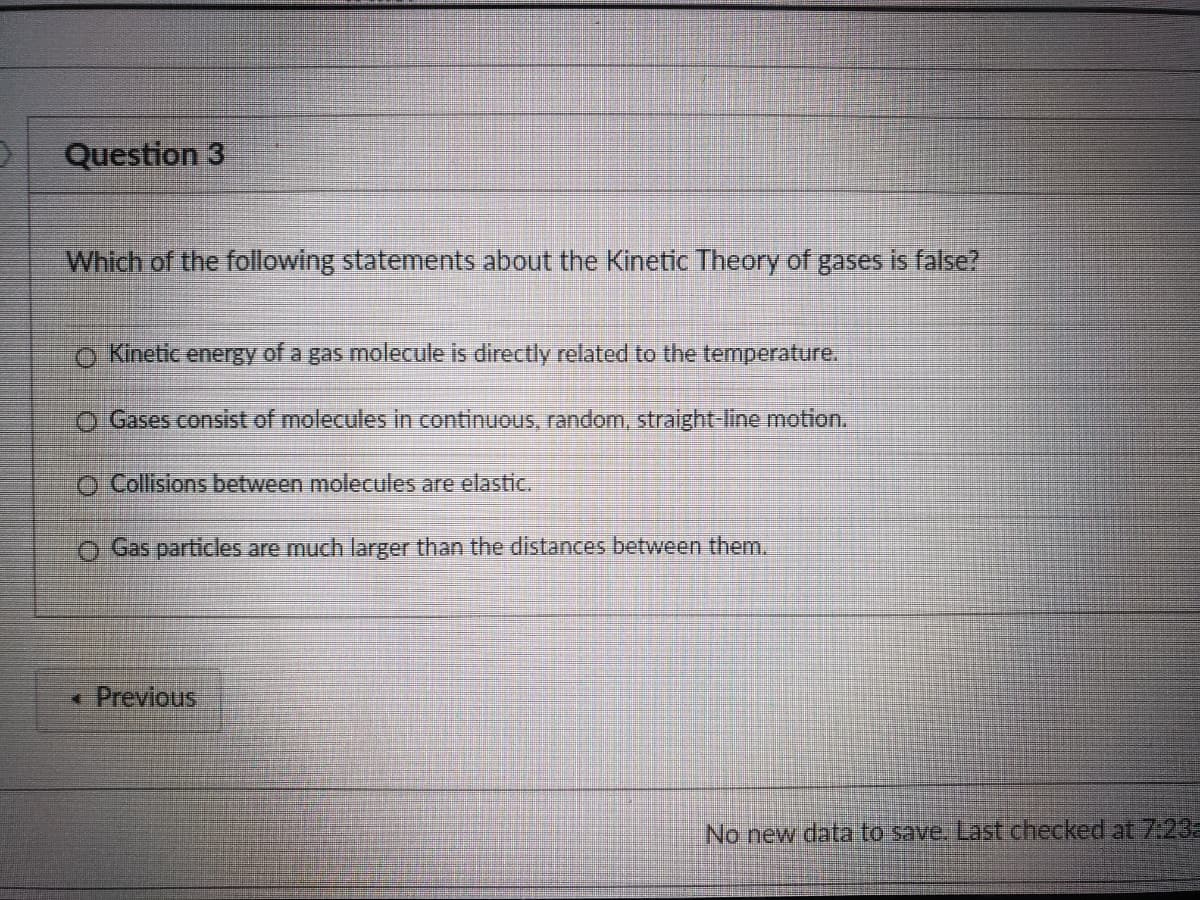
Which of the following statements about the Kinetic Theory of gases is false?
o Kinetic energy of a gas molecule is directly related to the temperature.
O Gases consist of molecules in continuous, random, straight-line motion.
O Collisions between molecules are elastic.
O Gas particles are much larger than the distances between them.
* Previous
No new data to save. Last checked at723
Expert Solution
Step 1
Kinetic molecular theory of gases:
Postulates are
1.kinetic energy of a molecule is directly proportional to absolute temperature
2.gaseous molecules move randomly in straight line in all the direction and at various speed.
3.collision between the molecules are perfectly elastic heart that is no energy to loss on collision due to friction
4.The average distance between the molecule is larger compared to diameter of the particle.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning