Which of the following would you expect to be a molecule and which would you expect to be part of an ionic solid? Drag the appropriate compounds to their respective bins. • View Available Hint(s) Reset Help Cas so KF co|KCI Nal Mgo HCI Molecular lonic Which of the following would you expect to be a molecule and which would you expect to be part of an ionic solid? Drag the appropriate compounds to their respective bins. • View Available Hint(s) Reset Help Cas so KF co|KCI Nal Mgo HCI Molecular lonic
Which of the following would you expect to be a molecule and which would you expect to be part of an ionic solid? Drag the appropriate compounds to their respective bins. • View Available Hint(s) Reset Help Cas so KF co|KCI Nal Mgo HCI Molecular lonic Which of the following would you expect to be a molecule and which would you expect to be part of an ionic solid? Drag the appropriate compounds to their respective bins. • View Available Hint(s) Reset Help Cas so KF co|KCI Nal Mgo HCI Molecular lonic
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter14: Liquids And Solids
Section: Chapter Questions
Problem 13QAP: The following data have ban collected for substance X. Construct. a heating curve for substance X....
Related questions
Question
- A covalent bond occurs when atoms share electrons. The resulting cluster of atoms is called a molecule, and the compound is considered to be molecular.
- An ionic bond results from a transfer of one or more electrons from one atom to another. This creates oppositely charged ions that group together into large macroscopic lattices as opposed to small clusters. For this reason, ionic compounds are not considered to be molecular.
As a general rule, covalent bonds occur between two nonmetal atoms whereas ionic bonds occur between a metal and a nonmetal atom.
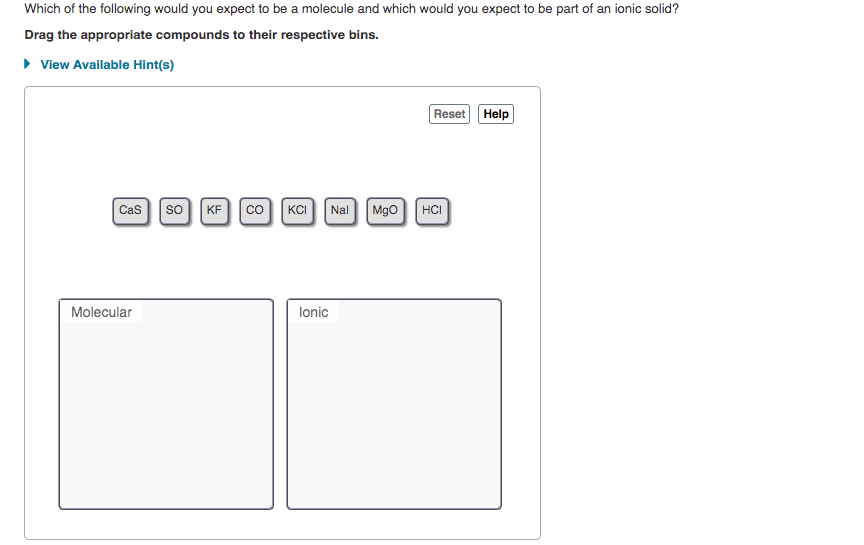
Transcribed Image Text:Which of the following would you expect to be a molecule and which would you expect to be part of an ionic solid?
Drag the appropriate compounds to their respective bins.
• View Available Hint(s)
Reset Help
Cas so
KF co|KCI Nal Mgo HCI
Molecular
lonic
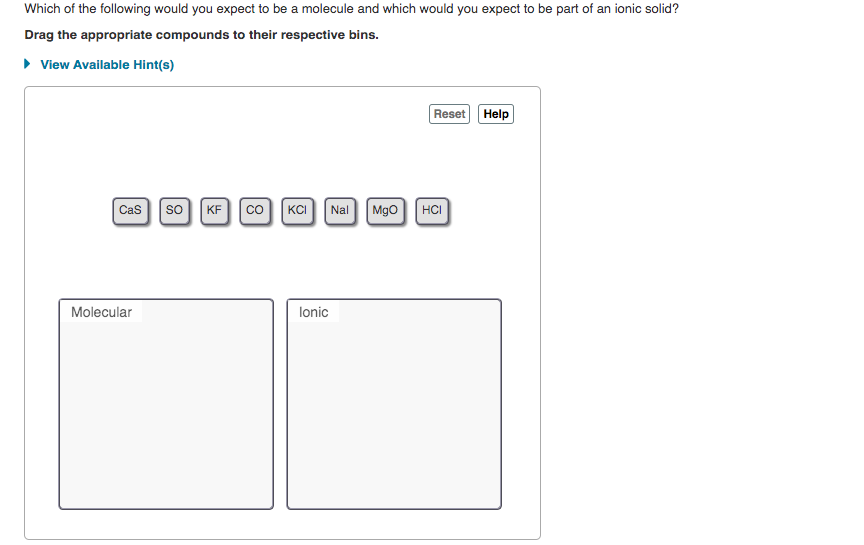
Transcribed Image Text:Which of the following would you expect to be a molecule and which would you expect to be part of an ionic solid?
Drag the appropriate compounds to their respective bins.
• View Available Hint(s)
Reset Help
Cas so
KF co|KCI Nal Mgo HCI
Molecular
lonic
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax