Which one of the following is not correct for the ESI spectrum of acebutolol? Select one: a. m/z at 318 corresponds to isotopic ion due to 18 atoms of 12C b. Molecular weight of the acebutolol is 337.2 gr C. + charged species are formed in acidic solution O d. There is not extensive fragmentation of the protonated ion e. m/z at 319.2 corresponds to isotopic ion due to 18 atoms of 13C O O
Which one of the following is not correct for the ESI spectrum of acebutolol? Select one: a. m/z at 318 corresponds to isotopic ion due to 18 atoms of 12C b. Molecular weight of the acebutolol is 337.2 gr C. + charged species are formed in acidic solution O d. There is not extensive fragmentation of the protonated ion e. m/z at 319.2 corresponds to isotopic ion due to 18 atoms of 13C O O
Chapter12: Structure Determination: Mass Spectrometry And Infrared Spectroscopy
Section12.SE: Something Extra
Problem 48AP: The infrared spectrum of the compound with the mass spectrum shown below lacks any significant...
Related questions
Question
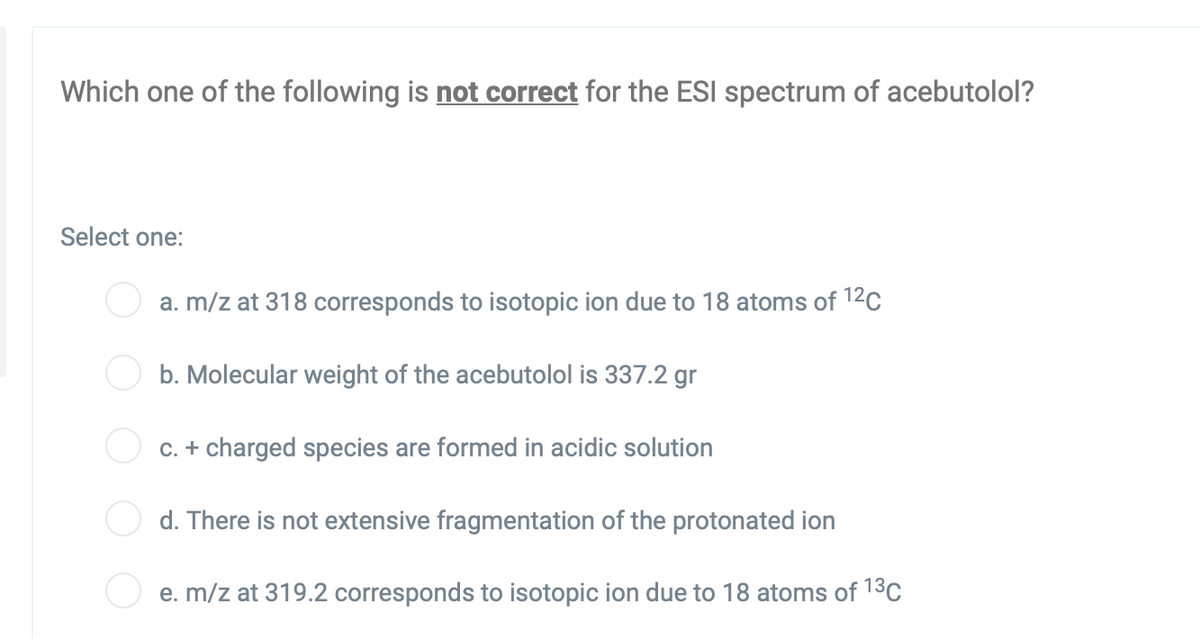
Transcribed Image Text:Which one of the following is not correct for the ESI spectrum of acebutolol?
Select one:
a. m/z at 318 corresponds to isotopic ion due to 18 atoms of 12c
b. Molecular weight of the acebutolol is 337.2 gr
C. + charged species are formed in acidic solution
d. There is not extensive fragmentation of the protonated ion
e. m/z at 319.2 corresponds to isotopic ion due to 18 atoms of 13C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

