The two mass spectra below correspond to two isomers of C4H10O: 1-butanol and 2-butanol. Match the spectrum with the appropriate compound. Place the m/z ratio and the structures for the labeled fragments in the table below. 100- Compound 00 GO 1 40 20- o ... p..r 10 20 30 40 50 60 70 80 90 100 m/7 100 - Compound 3 80 60 40 20 - 10 15 20 25 30 35 40 45 50 55 60 65 70 75 m/z Fragment 1 Fragment 2 Fragment 3 Fragment 4 m/z Fragment Relative Intensity Re-ative Intersity F8
The two mass spectra below correspond to two isomers of C4H10O: 1-butanol and 2-butanol. Match the spectrum with the appropriate compound. Place the m/z ratio and the structures for the labeled fragments in the table below. 100- Compound 00 GO 1 40 20- o ... p..r 10 20 30 40 50 60 70 80 90 100 m/7 100 - Compound 3 80 60 40 20 - 10 15 20 25 30 35 40 45 50 55 60 65 70 75 m/z Fragment 1 Fragment 2 Fragment 3 Fragment 4 m/z Fragment Relative Intensity Re-ative Intersity F8
Chapter12: Structure Determination: Mass Spectrometry And Infrared Spectroscopy
Section12.SE: Something Extra
Problem 48AP: The infrared spectrum of the compound with the mass spectrum shown below lacks any significant...
Related questions
Question
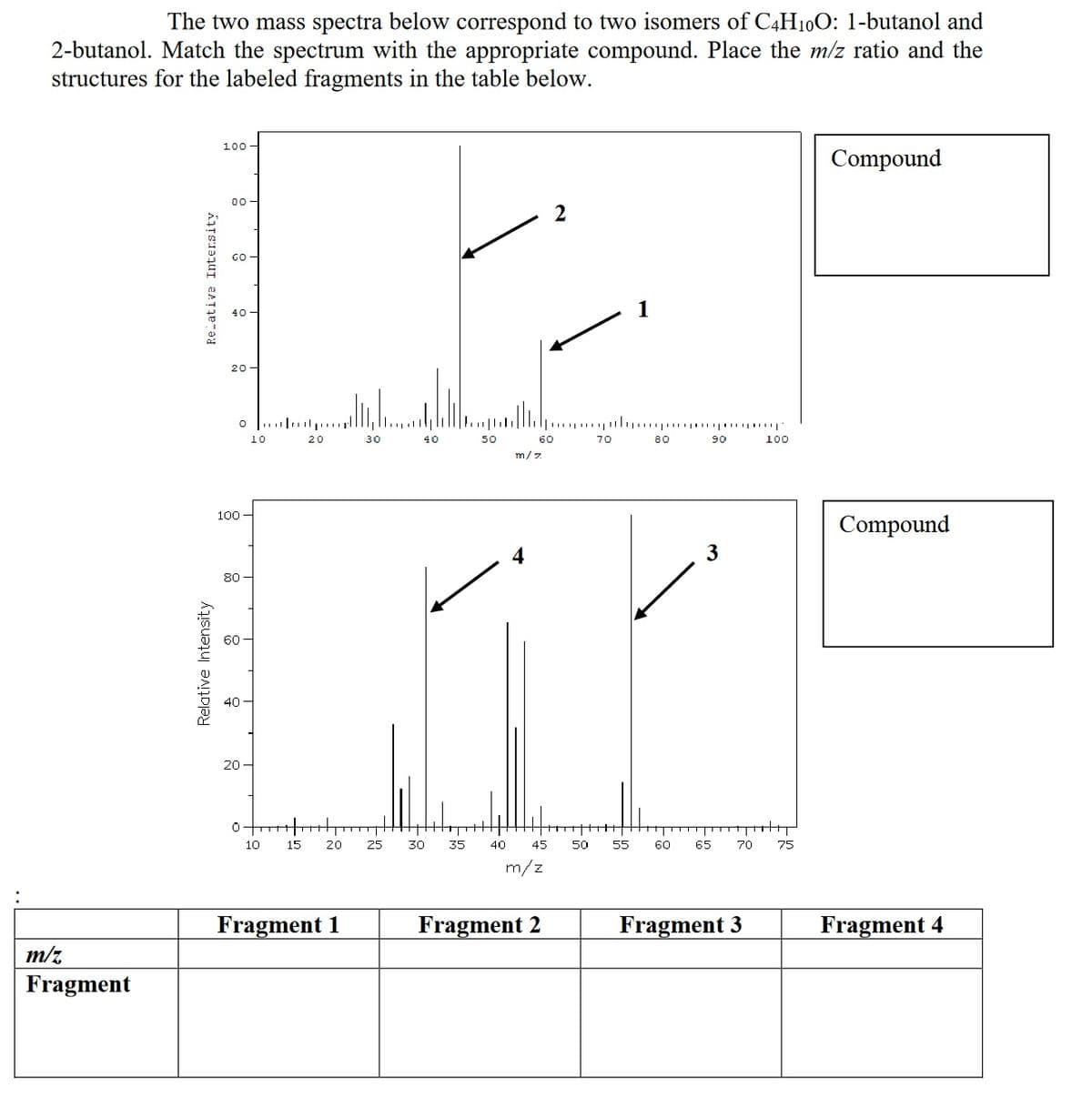
Transcribed Image Text:The two mass spectra below correspond to two isomers of C4H10O: 1-butanol and
2-butanol. Match the spectrum with the appropriate compound. Place the m/z ratio and the
structures for the labeled fragments in the table below.
100
Compound
00
2
GO
40 -
1
20 -
10
20
30
40
50
60
70
80
90
100
m/ 7
100
Compound
3
80
60
40
20
0 TH
10
15
20
25
30
35
40
45
50
55
60
65
70
75
m/z
Fragment 1
Fragment 2
Fragment 3
Fragment 4
m/z
Fragment
Relative Intensity
Re-ative Intersity
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning