Which statements are true about the p orbital shown? 1. It can hold up to two electrons. 2. It can hold up to six electrons. 3. An electron in this orbital moves around the edge in a figure eight pattern. 4. An electron of a certain energy is highly likely to be found within the orbital, but we cann exactly where. 1 and 1
Which statements are true about the p orbital shown? 1. It can hold up to two electrons. 2. It can hold up to six electrons. 3. An electron in this orbital moves around the edge in a figure eight pattern. 4. An electron of a certain energy is highly likely to be found within the orbital, but we cann exactly where. 1 and 1
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter7: The Structure Of Atoms And Periodic Trends
Section: Chapter Questions
Problem 67SCQ: Compare the configurations below with two electrons located in p orbitals. Which would be the most...
Related questions
Question
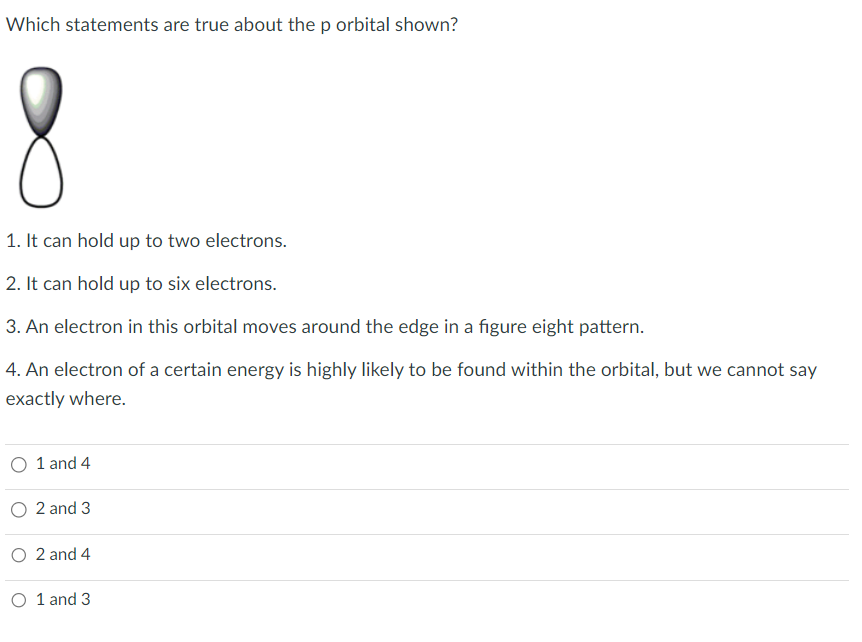
Transcribed Image Text:Which statements are true about the p orbital shown?
1. It can hold up to two electrons.
2. It can hold up to six electrons.
3. An electron in this orbital moves around the edge in a figure eight pattern.
4. An electron of a certain energy is highly likely to be found within the orbital, but we cannot say
exactly where.
O 1 and 4
O 2 and 3
O 2 and 4
O 1 and 3

Transcribed Image Text:What type of bonding would most likely give you the following properties?
Does not conduct electricity
▪ Solid at room temperature but easily changes into its gaseous state
▪ Boiling point 457K
I
▪ Melting point 387K
O Metallic bonding
O Network of covalent bonds (i.e. diamond)
O lonic bonding
O Molecules that contain covalent bonds (i.e. F₂)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co