white powder i-i red blue 4a What event is occuring? Sa. What event is occuring? 6a What event is occuring? 46. Circle 5b. Circle: 6b. Circle Chemical Change or Physical Change Chemical Change or Physical Change Chermical Change or Physical Change magnesium ribbon bright light liquid A 25C water liquid B 25°C 7a What event is occuring? 8a. What event is occuring? 9a. What event is occuring? 7b. Circle 8b. Circle 9ь. Circle Chemical Change or Physical Change Chemical Change or Physical Change Chemical Change Physical Change 10. Give one more example of a chemical change, a chemical change you might witness in lab but is NOT pictured in a diagram above!
white powder i-i red blue 4a What event is occuring? Sa. What event is occuring? 6a What event is occuring? 46. Circle 5b. Circle: 6b. Circle Chemical Change or Physical Change Chemical Change or Physical Change Chermical Change or Physical Change magnesium ribbon bright light liquid A 25C water liquid B 25°C 7a What event is occuring? 8a. What event is occuring? 9a. What event is occuring? 7b. Circle 8b. Circle 9ь. Circle Chemical Change or Physical Change Chemical Change or Physical Change Chemical Change Physical Change 10. Give one more example of a chemical change, a chemical change you might witness in lab but is NOT pictured in a diagram above!
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter6: Thermochemisty
Section: Chapter Questions
Problem 6.30QP: Enthalpy a A 100.-g sample of water is placed in an insulated container and allowed to come to room...
Related questions
Question
100%
Hi can you please help me with my chemistry homework this due today and I’m so confused thank you so much I really appreciate your help
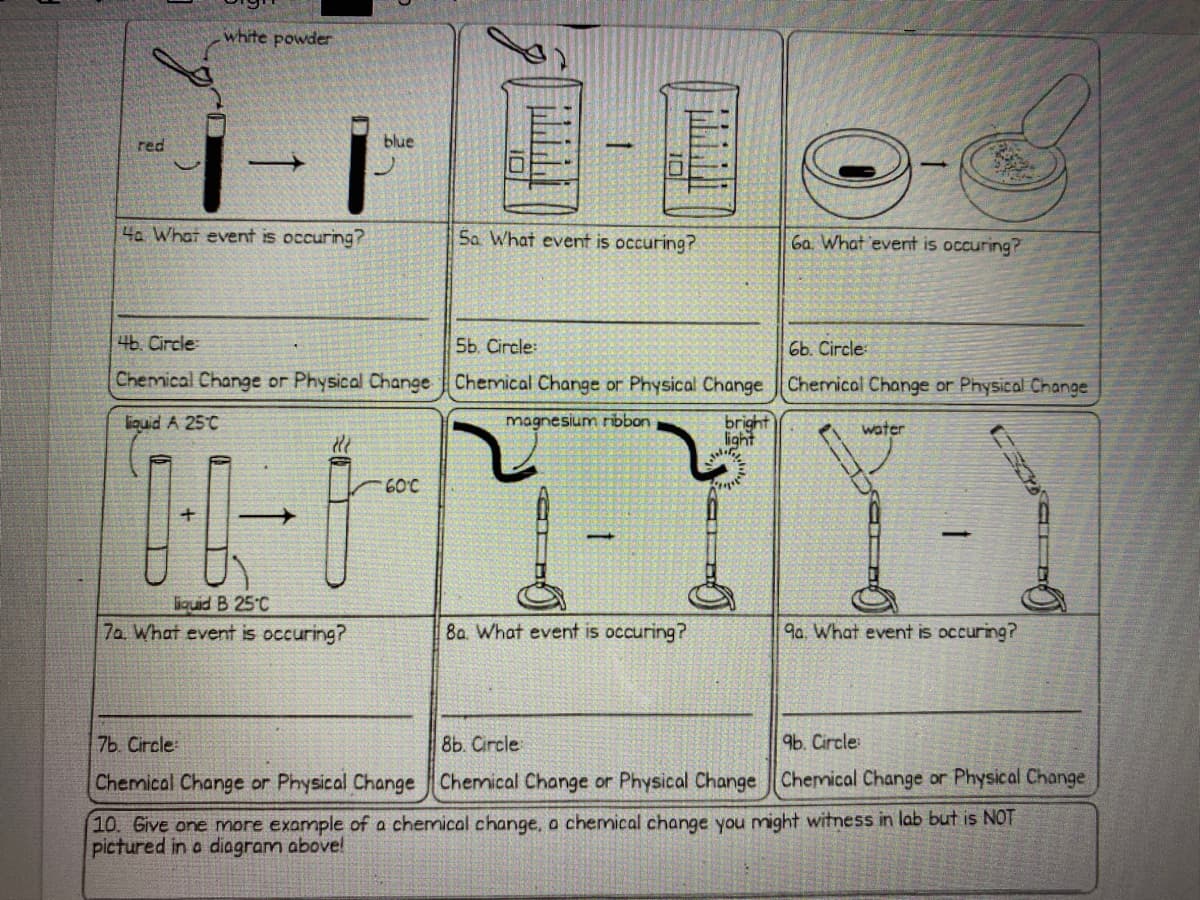
Transcribed Image Text:white powder
i-i
red
blue
4a What event is occuring?
Sa What event is occuring?
6a What event is occuring?
4b. Circle
5b. Circle:
6b. Circle
Chemical Change or Physical Change Chemical Change or Physical Change Chermical Change or Physical Change
bright
light
liouid A 25°C
magnesium ribbon
water
60°C
liquid B 25°C
7a What event is occuring?
8a. What event is occuring?
9a. What event is occuring?
7b. Circle
8b. Circle
96. Circle:
Chemical Change or Physical Change Chemical Change or Physical Change Chemical Change or Physical Change
10. Give one more example of a chemical change, a chemical change you might witness in lab but is NOT
pictured in a diagram above!
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning