Why is the alkyl bromide substrate below not capable of undergoing an E2 elimination reaction upon treatment with potassium hydroxide (KOH) in ethanol (EtOH)? -Br– is too poor a leaving group. -Too much angle strain would be present in the alkene product. -Potassium hydroxide is a poor base to use in E2 reactions. -An anti-periplanar E2 elimination cannot occur due to the lack of a beta-hydrogen in the substrate
Why is the alkyl bromide substrate below not capable of undergoing an E2 elimination reaction upon treatment with potassium hydroxide (KOH) in ethanol (EtOH)? -Br– is too poor a leaving group. -Too much angle strain would be present in the alkene product. -Potassium hydroxide is a poor base to use in E2 reactions. -An anti-periplanar E2 elimination cannot occur due to the lack of a beta-hydrogen in the substrate
Chapter3: Mechanisms
Section: Chapter Questions
Problem 96EQ
Related questions
Question
Why is the alkyl bromide substrate below not capable of undergoing an E2 elimination reaction upon treatment with potassium hydroxide (KOH) in ethanol (EtOH)?
-Br– is too poor a leaving group.
-Too much angle strain would be present in the alkene product.
-Potassium hydroxide is a poor base to use in E2 reactions.
-An anti-periplanar E2 elimination cannot occur due to the lack of a beta-hydrogen in the substrate
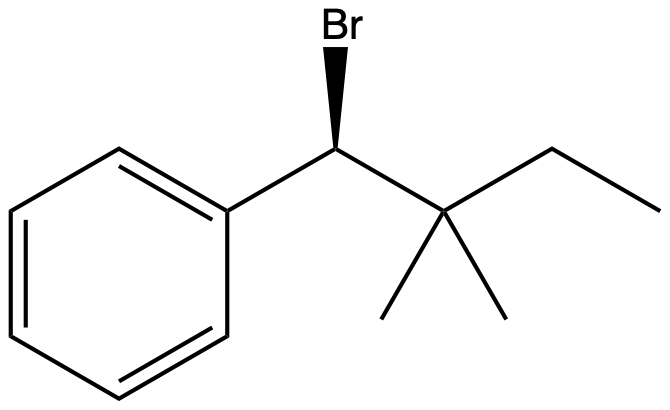
Transcribed Image Text:Br
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

