Will acetylene react with sodium hydride according to the following equation to form a salt and hydrogen, H,? Using pK, values given in Table 4.1, calculate K for this equilibrium. HC=CH + Na H- HC=C Na+ + H, Acetylene Sodium Sodium Hydrogen hydride acetylide
Will acetylene react with sodium hydride according to the following equation to form a salt and hydrogen, H,? Using pK, values given in Table 4.1, calculate K for this equilibrium. HC=CH + Na H- HC=C Na+ + H, Acetylene Sodium Sodium Hydrogen hydride acetylide
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter19: Enolate Anions And Enamines
Section19.9: Crossed Enolate Reactions Using Lda
Problem JQ
Related questions
Question
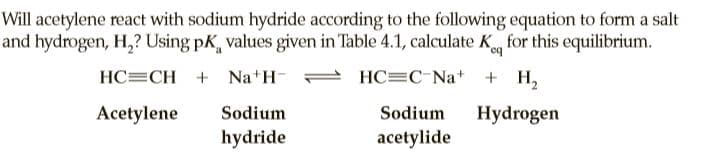
Transcribed Image Text:Will acetylene react with sodium hydride according to the following equation to form a salt
and hydrogen, H,? Using pK, values given in Table 4.1, calculate K for this equilibrium.
HC=CH + Na H-
HC=C Na+
+ H,
Acetylene
Sodium
Sodium
Hydrogen
hydride
acetylide
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT