with ammonia (NH,). Ammonium phosphate ((NH,),PO,) is an important ingredient in many fertilizers. It can be made by reacting phosphoric acid (H, PO What mass of ammonium phosphate is produced by the reaction of 6.3 g of ammonia? Round your answer to 2 significant digits.
with ammonia (NH,). Ammonium phosphate ((NH,),PO,) is an important ingredient in many fertilizers. It can be made by reacting phosphoric acid (H, PO What mass of ammonium phosphate is produced by the reaction of 6.3 g of ammonia? Round your answer to 2 significant digits.
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter1: Basic Concepts Of Chemistry
Section: Chapter Questions
Problem 28RPS: Use the following graph to answer the following question: (a) What is the value of x when y=4.0? (b)...
Related questions
Question
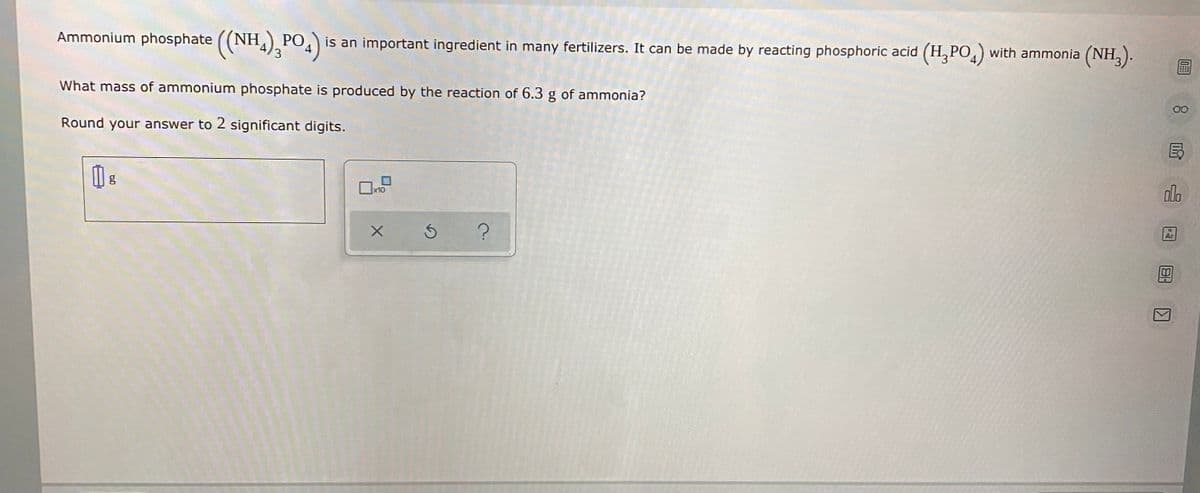
Transcribed Image Text:Ammonium phosphate ((NH,). PO) is an important ingredient in many fertilizers. It can be made by reacting phosphoric acid (H, PO,) with ammonia (NH,).
What mass of ammonium phosphate is produced by the reaction of 6.3 g of ammonia?
00
Round your answer to 2 significant digits.
民
x10
ola
Ar
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning