With the information from my pictures please help me with the following: 1. Volume at Equivalence Point_______ 2. Volume at one-half Equivalence Point _________ 3. pKa________ 4. Ka_________ 5. Average Ka (show Calculations)_________ 6. Standard Deviation of Ka _________
With the information from my pictures please help me with the following: 1. Volume at Equivalence Point_______ 2. Volume at one-half Equivalence Point _________ 3. pKa________ 4. Ka_________ 5. Average Ka (show Calculations)_________ 6. Standard Deviation of Ka _________
Chapter21: Potentiometry
Section: Chapter Questions
Problem 21.22QAP
Related questions
Question
With the information from my pictures please help me with the following:
1. Volume at Equivalence Point_______
2. Volume at one-half Equivalence Point _________
3. pKa________
4. Ka_________
5. Average Ka (show Calculations)_________
6. Standard Deviation of Ka _________
If I need to I will post 6 different posts.
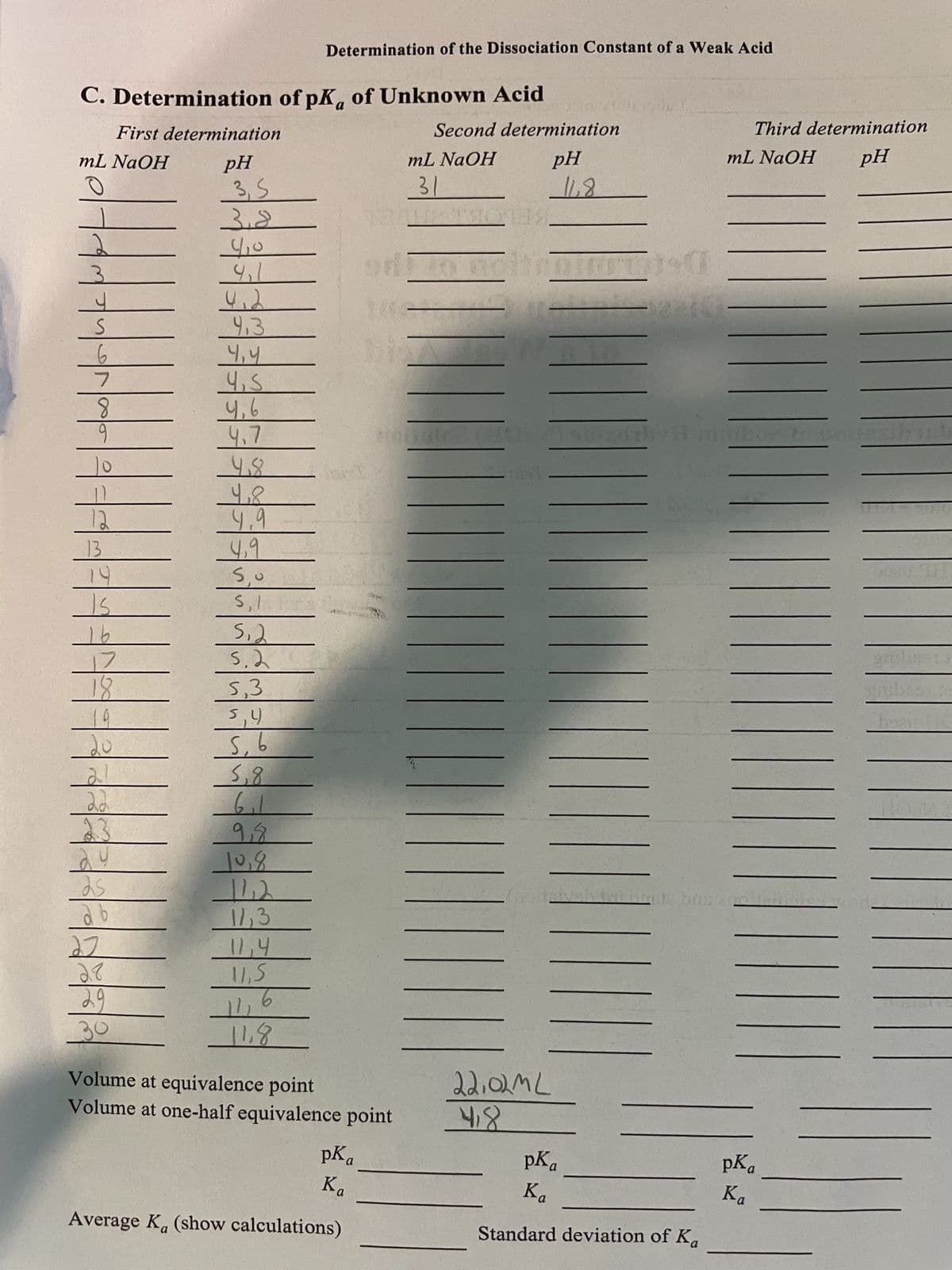
Transcribed Image Text:C. Determination of pK, of Unknown Acid
First determination
mL NaOH
pH
mL NaOH
31
Ի
S
6
8
9.
13
19
ST
1.6
17
18
_19
20
22
44
he
25
a b
се
3, S
3,8
9₁1
412
9.3.
4,4
4,5
4,6
4.7
9.8
4.8
4,9
4,9
c's
I's
e's
5.2
5,3
hi's
5, 6
85
6₁1
9,8
10,8
-142
11,3
Determination of the Dissociation Constant of a Weak Acid
Second determination
pH
h'll
BELORESTEL
DOCCLINTOJIO OFIDG
De
TH
Terar
28
11,5
6
29
30
-11,8
Volume at equivalence point
Volume at one-half equivalence point
pKa
Ka
Average Ka (show calculations)
110
20
BAGEOR
Third determination
pH
22,02ML
418
pKa
Ka
Standard deviation of Ka
mL NaOH
50
hente ons al
pka
Ka
gribe
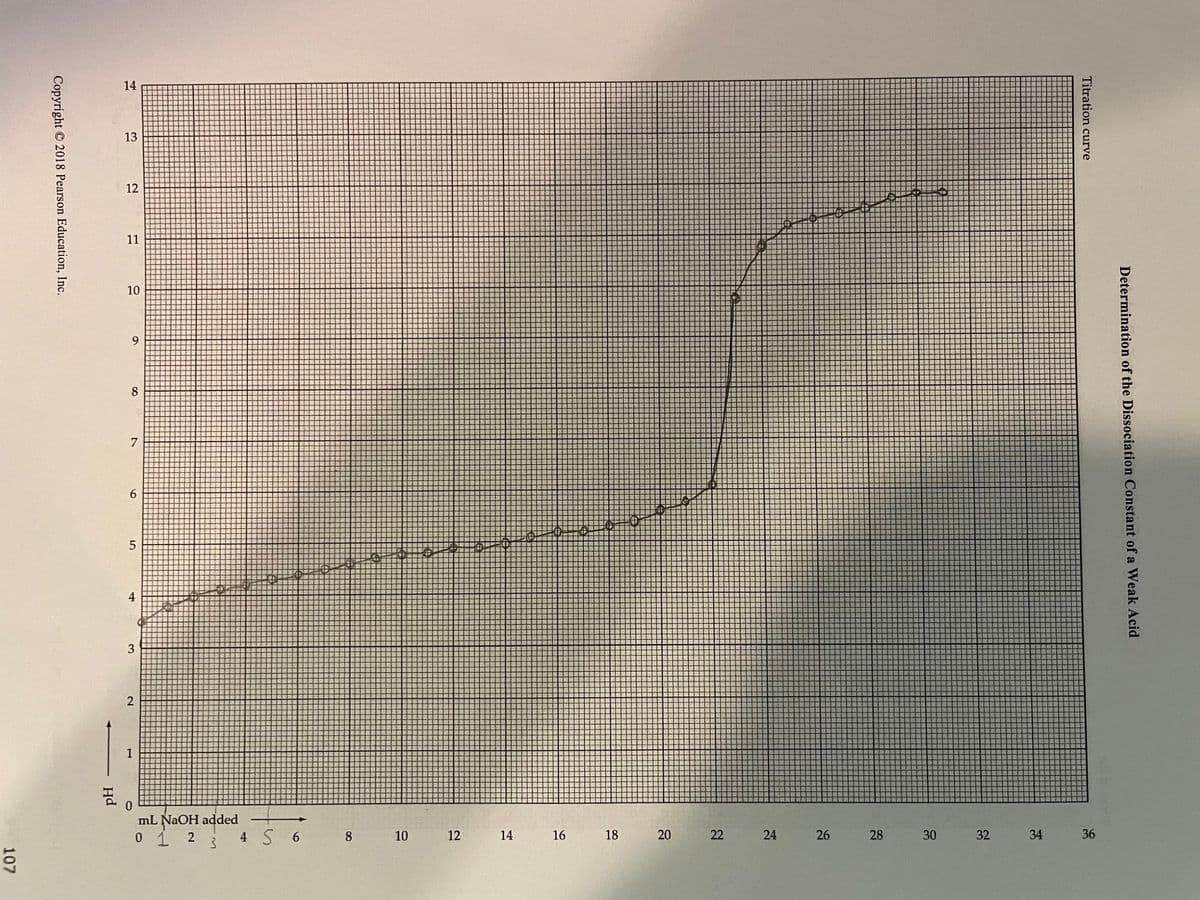
Transcribed Image Text:107
Copyright 2018 Pearson Education, Inc.
Bry
Hd
14
13
12
11
10
9
8
7
6
5
4
3
2
T
0
ml NaOH added
0 1 2 3 4 5 6 8
10
12
14
16
18
20
22
24
26
28
30
32
34
Titration curve
36
Determination of the Dissociation Constant of a Weak Acid
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning