Wittig Reaction HW4. Study the following ylide used in the reaction. Is the circled ester group electron donating or electron withdrawing? A 8- 8+ PPh3 OMe B HW5. Relative to the partially negative carbon nucleophile, is the ester group stabilizing the partial negative charge or destabilizing the partial negative charge? electron HW6. Study the following ylide. Is the circled methyl group electron donating or electron withdrawing? CH3 8- 8+ PPh3 group electron group HW7. Relative to the partially negative carbon nucleophile of the ylide, is the methyl group stabilizing or destabilizing? HW8. In the proper solvent systems, which ylide (A or B) would you expect to react faster?
Wittig Reaction HW4. Study the following ylide used in the reaction. Is the circled ester group electron donating or electron withdrawing? A 8- 8+ PPh3 OMe B HW5. Relative to the partially negative carbon nucleophile, is the ester group stabilizing the partial negative charge or destabilizing the partial negative charge? electron HW6. Study the following ylide. Is the circled methyl group electron donating or electron withdrawing? CH3 8- 8+ PPh3 group electron group HW7. Relative to the partially negative carbon nucleophile of the ylide, is the methyl group stabilizing or destabilizing? HW8. In the proper solvent systems, which ylide (A or B) would you expect to react faster?
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter16: Synthesis Workshop 1
Section: Chapter Questions
Problem 21CTQ
Related questions
Question
please answer these question regarding figures A and B
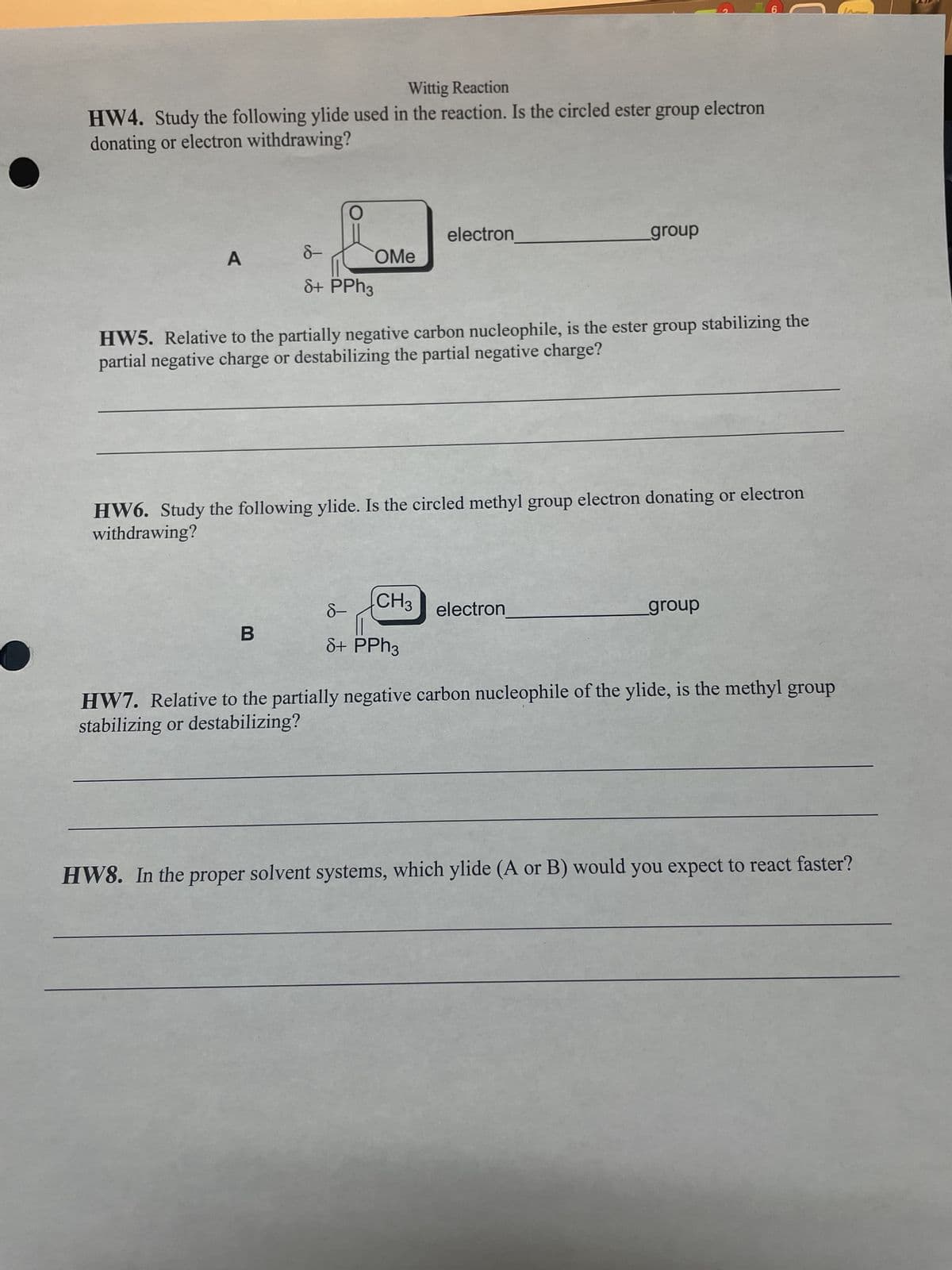
Transcribed Image Text:Wittig Reaction
HW4. Study the following ylide used in the reaction. Is the circled ester group electron
donating or electron withdrawing?
is t
O
B
8+ PPh3
OMe
electron
HW5. Relative to the partially negative carbon nucleophile, is the ester group stabilizing the
partial negative charge or destabilizing the partial negative charge?
CH3
HW6. Study the following ylide. Is the circled methyl group electron donating or electron
withdrawing?
8-
8+ PPh3
group
electron
6
group
HW7. Relative to the partially negative carbon nucleophile of the ylide, is the methyl group
stabilizing or destabilizing?
HW8. In the proper solvent systems, which ylide (A or B) would you expect to react faster?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning



Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning



EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT