Worksheet 1.1 Introduction to Atomic Theory (Application of knowledge) READ PAGES 8-28 IN THE TEXTBOOK BEFORE ANSWERING THESE QUESTIONS and watch the following videos https://www.youtube.com/watch?y=SKKsLuRPsyU https://www.youtube.com/watch?y=fkpZfpNWjWY&t=110s 1. List the principles of Dalton's atomic theory. 2. After reading pages 8-28, fill in the table below.
Worksheet 1.1 Introduction to Atomic Theory (Application of knowledge) READ PAGES 8-28 IN THE TEXTBOOK BEFORE ANSWERING THESE QUESTIONS and watch the following videos https://www.youtube.com/watch?y=SKKsLuRPsyU https://www.youtube.com/watch?y=fkpZfpNWjWY&t=110s 1. List the principles of Dalton's atomic theory. 2. After reading pages 8-28, fill in the table below.
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 18Q: Section 1-5 describes the postulates of Daltons atomic theory. With some modifications, these...
Related questions
Question
Please help me comple this!
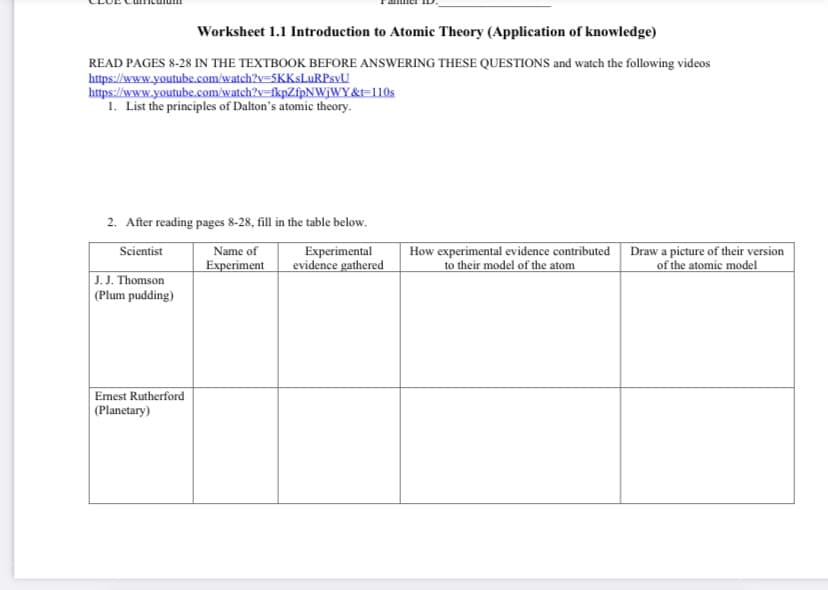
Transcribed Image Text:Worksheet 1.1 Introduction to Atomic Theory (Application of knowledge)
READ PAGES 8-28 IN THE TEXTBOOK BEFORE ANSWERING THESE QUESTIONS and watch the following videos
https://www.youtube.com/watch?v=5KKsLuRPsyU
https://www.youtube.com/watch?v=fkpZfpNWjWY&t=110s
1. List the principles of Dalton's atomic theory.
2. After reading pages 8-28, fill in the table below.
How experimental evidence contributed
to their model of the atom
Scientist
Experimental
evidence gathered
Draw a picture of their version
of the atomic model
Name of
Experiment
J. J. Thomson
|(Plum pudding)
Emest Rutherford
|(Planetary)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning