Write a balanced chemical equation for the given hydrate in question #1 to decompose into anhydrous salt and water. Hint: follow the example from the Introduction page in Lab 3. Pay attention to the states of matter.
Write a balanced chemical equation for the given hydrate in question #1 to decompose into anhydrous salt and water. Hint: follow the example from the Introduction page in Lab 3. Pay attention to the states of matter.
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter5: Nomenclature
Section: Chapter Questions
Problem 16CR
Related questions
Question
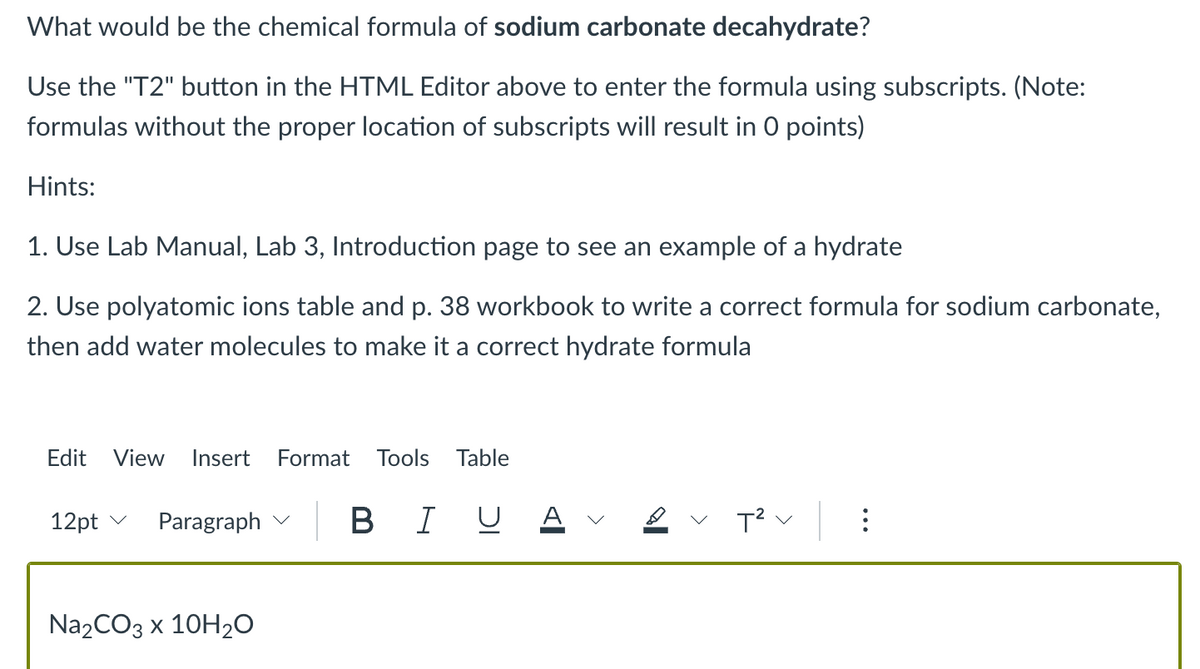
Transcribed Image Text:What would be the chemical formula of sodium carbonate decahydrate?
Use the "T2" button in the HTML Editor above to enter the formula using subscripts. (Note:
formulas without the proper location of subscripts will result in 0 points)
Hints:
1. Use Lab Manual, Lab 3, Introduction page to see an example of a hydrate
2. Use polyatomic ions table and p. 38 workbook to write a correct formula for sodium carbonate,
then add water molecules to make it a correct hydrate formula
Edit View Insert Format Tools Table
BI U
12pt ✓
Paragraph
Na₂CO3 x 10H₂O
T² ✓
⠀

Transcribed Image Text:Write a balanced chemical equation for the given hydrate in question #1 to decompose into
anhydrous salt and water. Hint: follow the example from the Introduction page in Lab 3. Pay
attention to the states of matter.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning