Write a balanced equation for the double-replacement neutralization reaction described, using the smallest possible integer coefficients. A reaction occurs when aqueous solutions of phosphoric acid and sodium hydroxide are combined. Assume excess base. Write a balanced equation for the double-replacement neutralization reaction described, using the smallest possible integer coefficients. A reaction occurs when aqueous solutions of calcium hydroxide and hydrochloric acid are combined
Write a balanced equation for the double-replacement neutralization reaction described, using the smallest possible integer coefficients. A reaction occurs when aqueous solutions of phosphoric acid and sodium hydroxide are combined. Assume excess base. Write a balanced equation for the double-replacement neutralization reaction described, using the smallest possible integer coefficients. A reaction occurs when aqueous solutions of calcium hydroxide and hydrochloric acid are combined
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter17: Acid-base(proton Transfer) Reactions
Section: Chapter Questions
Problem 12E
Related questions
Question
- Write a balanced equation for the double-replacement neutralization reaction described, using the smallest possible integer coefficients. A reaction occurs when aqueous solutions of phosphoric acid and sodium hydroxide are combined. Assume excess base.
- Write a balanced equation for the double-replacement neutralization reaction described, using the smallest possible integer coefficients.
A reaction occurs when aqueous solutions of calcium hydroxide and hydrochloric acid are combined.
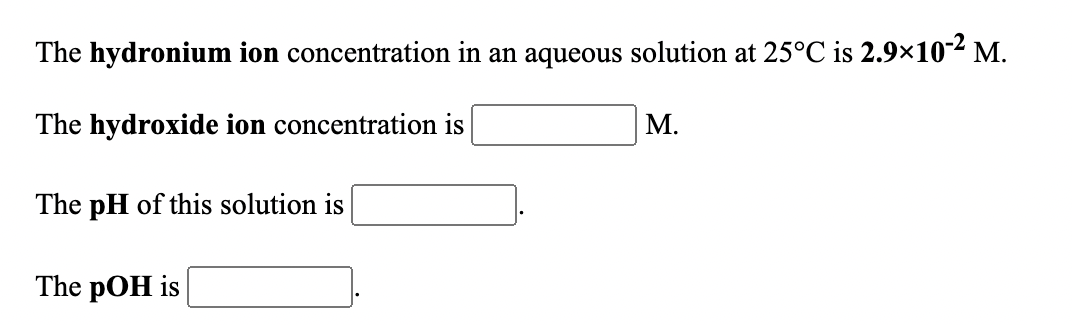
Transcribed Image Text:The hydronium ion concentration in an aqueous solution at 25°C is 2.9×10² M.
The hydroxide ion concentration is
М.
The pH of this solution is
The pOH is
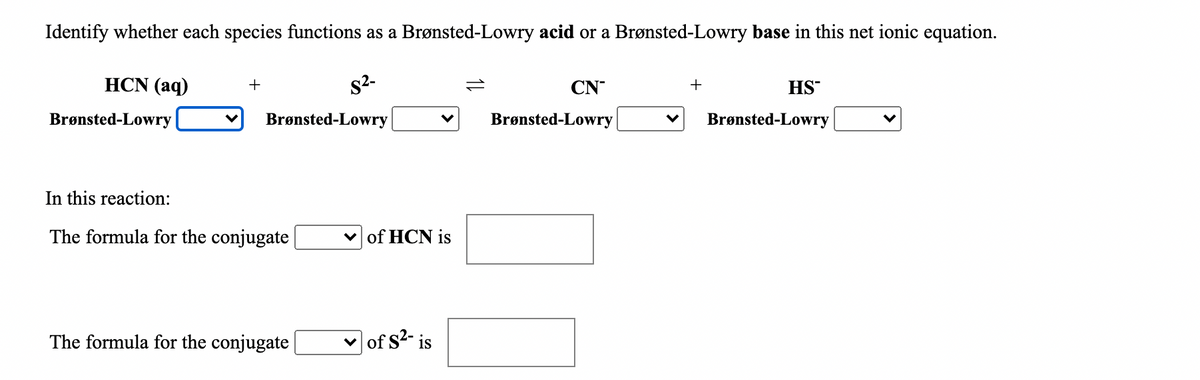
Transcribed Image Text:Identify whether each species functions as a Brønsted-Lowry acid or a Brønsted-Lowry base in this net ionic equation.
HCN (aq)
s2-
+
1L
CN
+
HS
Brønsted-Lowry
Brønsted-Lowry
Brønsted-Lowry
Brønsted-Lowry
In this reaction:
The formula for the conjugate |
v of HCN is
The formula for the conjugate
v of s2- is
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning