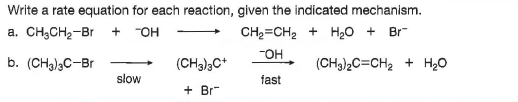
Write a rate equation for each reaction, given the indicated mechanism. a. CH,CH2-Br + "OH CH2=CH2 + H20 + Br -OH b. (CH3)3C-Br (CH3)3C+ (CH3)2C=CH2 + H20 slow fast + Br
Q: Which one of the following is the best(faster reaction) nucleophile for the 2-bromobutane SN1…
A: the strength of nucleophile does not affect the reaction rate of SN1 because the nucleophile is not…
Q: Choose the reagent(s) that would be most likely to complete this reaction. Br2 (1 equiv) H2O Br
A:
Q: Which reaction schemes is best to give the product? HO. NO2 O The following reaction scheme: CH3…
A:
Q: 1. For each of the following pairs of SN2 reactions, indicate which reaction occurs faster: a)…
A:
Q: ach reaction box, place the best reagent and conditions from the list below. 1) 2) но 3) но. 4)…
A: Given reactant is : 3-methyl-3-pentanol.
Q: Using the reaction bank, devise a synthetic route (should use several reactions) to convert the…
A: Given organic reaction.
Q: A set of three nucleophilic displacement reactions is shown below: CH3 SOH CH3CH2CCH3 SN1 reaction…
A: The replacement of any atom or group of atoms by a nucleophile is termed as nucleophilic…
Q: Select the major product of the reaction sequence below. OH OH CH,CH,CCN CH,CH3 CH,CH,CCH,CH, CO,H B…
A: Acyl chlorides are also called acid chlorides, with the general formula of RCOCl, the R represents…
Q: The fastest method of synthesis of isopropyl methyl ether (below) is: CH3 CH3 CH-0 H3C CH. H;C…
A: The solution is given below -
Q: a. What are the products of following reactions? b. Write the reaction mechanism for each using the…
A:
Q: 3. (10 points) Complete the reaction scheme below. Show all reagents and intermediates. No reaction…
A:
Q: In each reaction box, place the best reagent and conditions from the list below. 1) 2) Br 3) Br2…
A: Friedel-Crafts alkylation can be done in the first step and the reagent ethylchloride in presence of…
Q: CH3 SOH CH;CH,CCH3 SN1 reaction Br A, SOH = 100% CH3OH %3D B, SOH = 33% CH3OH, 67% H20 C, SOH = 67%…
A:
Q: Br CH3-CEC -CH3 2HB1 CH3-Č-CH2-CH3 + Br
A:
Q: GS Q5 Show all missing reagents and intermediates in the reaction sequence below. Put the…
A:
Q: Which reaction schemes is best to give the product? 2. HO. NO 2 O The following reaction scheme: Br2…
A:
Q: Which is the proper reaction mechanism for the following reaction? F OT F-B F :BF, CH2CH, CH2CH3…
A: Option II
Q: 1 reaction is each pair is faster? CH;OH CH;OH Br CH CH,OH Br OMSO Br CH,CH OH ZO0
A:
Q: Arrange the reactants from fastest to slowest in an S№2 reaction. (CH3)2C(CI)CH3 CH3CH(CI)CH2CH3…
A:
Q: In each reaction box, place the best reagent and conditions from the list below. Br 1) Br 2) 3) 4)…
A: Given reaction:
Q: The following reaction takes place several times faster than the reaction of 2-chlorobutane with…
A: The enhances rate of reaction is due to presence of -N..Et2 which undergoes intro molecular SN2…
Q: Br CH3SH CH3SH h. Ph
A: In the questions ( H and M ), we will give Reaction mechanism and Final Product(s). You can see…
Q: In each reaction box, place the best reagent and conditions from the list below. 1) 2) 3) 4) CH3BR…
A:
Q: What is a major product of the reaction sequence shown in the box? Br2 ? -Br Pd(OAc)2, K2CO3 Bu4NBr,…
A:
Q: Choose the reagent(s) that would be most likely to complete this reaction. HBr (1 equiv) H2O2
A:
Q: Using the reaction bank, devise a synthetic route (should use several reactions) to convert the…
A:
Q: In each reaction box, place the best reagent and conditions from the list below. 1) 2) Br 3)…
A: In the given reaction, propyl (alkyl) and bromo group has to be introduced to the benzene ring.…
Q: Fill in the missing reagent(s) for each step. (Note: subscripts/superscripts are not supported in…
A: Alcohol is an organic compound with at least one hydroxyl functional group attached to a saturated…
Q: Determine reagents C and D from the reaction scheme below. Choose two (2) answers. Select one or…
A:
Q: What is the rate expression for this reaction? CH3-CH2-Br + CH3-NH2 polar aprotic solvent (A) rate =…
A: Given reaction: CH3CH2Br + CH3NH2 →polar aprotic solvent In order to find the rate expression for…
Q: Using the reaction bank, devise a synthetic route (should use several reactions) to convert the…
A: Given
Q: For the given reaction, use the identity of the alkyl halide and nucleophile to determine which…
A: The given reaction uses secondary halide as the reactant, -OCH3 as the nucleophile, and CH3OH or…
Q: What is the product or mixture of products A expected from the reaction below: Br2 FeBrz CH2- Br-…
A: In the organic conversion, suitable reagent converts the reactant into products. Reagents attacks…
Q: CH, KOCH 3)3 H3C- -CH3 Solvent: Toluene HCI CH3 HO. CH3 Solvent: Toluene H3C
A: Here, first reaction is elimination and second reaction is substitution reaction
Q: Arrow-pushing Instructions MeOH Draw curved arrows to show the movement of electrons in this step of…
A: Given reaction is : Draw the curved arrows to show the movement of electrons in this step of the…
Q: rite the mechanism for the reaction below. -OH + CH3OH
A:
Q: för the following transformation? A. 1 only B. 2 only C. 1 and 4 only D. 3 only но. 1. NANH, 2.…
A: Applying concept of reagents and chemical reaction.
Q: For the nitration of a monosubstituted benzene, which substituent, when attached to benzene,…
A: We add conc HNO3 and conc H2SO4 to bring about nitration in each of the species.
Q: Which statement is true for S2 reactions? a) Reaction rate depends on stability of carbocation b)…
A: True statement about Sn2 reaction is
Q: ? Br What is the best reagent for the transformation shown above? B E „Mgl A D PPH3
A: We know that R-Li gives exclusively gives 1,2 product.
Q: What is the rate law implied by the mechanism given below? CH3COCH3(aq) + H+(aq) ←→…
A: The rate law is a mathematical expression that relates concentration of reactants and the rate of a…
Q: Question 1 Consider the following reaction scheme and answer the questions that follow: он i. CH,CH…
A:
Q: Reagents 2 equivalents of NaNH2 H2, Lindlar's catalyst Na / NH3 a. HBr m. HBr, H2O2, hv c. H2O,…
A: We have to identify the reagents nessato accomplish the following given reaction as follows in step…
Q: 4. Circle the electrophilic and nucleophilic atoms in each substitution reaction below. Provide the…
A: We have to identify the electrophilic atom and the nucleophilic atom, also the stereochemistry of…
Q: b. Write the reaction mechanism for each using the right arrows and define it as Sn2, Sn1, E2 or E1.…
A:
Q: Which one of the following is the best(faster reaction) nucleophile for the 2-bromobutane SN2…
A:
Q: This reaction can be completed with which reagents? (CH)CHCH;CH;c=CH (CH;),CHCH,CH;CCH: O H20,…
A: Hydration of alkyne
Q: 3. What is the major product of this reaction sequence? CH-C%3С-НNaNH2, CH3B. H20, H2SO4 final II…
A:
Q: CH3 o-nitrotoluene CH3 NH, CI NO2 CH3 o-toluidine NH3*,CI CH3 CI 2-chloro-N-o-tolilpropanami NH,HCO,…
A: The solution is given below:
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

- Below is the equation for a nucleophilic substitution reaction and some experimental data. CH3CH2Br + CH3COO- ⇌ CH3CH2CO2CH3 + Br- ΔH=-75 kJ/mol Rate = k [CH3CH2Br][CH3COO-] Which reaction energy profile would be the best representative of the data provided?Give 3 examples of a reaction mechanism of E1 that follows Zaitsev's rule.What is the mechanism for the entire reaction below?
- For each reaction indicate the predominant mechanism: SN1, SN2, E1, or E2Below is the equation for a nucleophilic substitution reaction and some experimental data. CH3CH2Br + CH3COO- ⇌ CH3CH2CO2CH3 + Br- Rate = k [CH3CH2Br][CH3COO-] Which mechanism would best fit the data?Which overall reaction is consistent with the reaction mechanism below? (CH3)3CBr→(CH3)3C++Br- (CH3)3C++OH-→(CH3)3COH (CH3)3CBr+ OH-→(CH3)3COH+Br- (CH3)3C++Br-→(CH3)3COH (CH3)3CBr+OH-→(CH3)3C++(CH3)3COH (CH3)3C++OH-→(CH3)3COH
- What type of mechanism is exhibited in the reaction? SN1, SN2, E2, E1?Can someone please help me with 2 mechanisms? One is for the synthesis of 1-bromobutane, and the other is for 2-bromobutane. The attached reaction shows the reagents and starting product.-Name the correct mechanism via arrow pushing -name each mechanism -name each minor and major products (1R,2S)-1-iodo-2-methylcyclohexane + ethanol/DMSO/stirred 3 days
- For SN1 Explain the order in which 1o (primary) alkyl halides reacted (fastest to slowest) and explain why. The 1o primary alkyl halides are: (see picture below) 1-chlorobutane 1-bromobutane 1-chloro-2butene benzylchlorideThe substitution of an Iby a Clon H3C-I can occur by two possiblemechanisms:Mechanism I: step 1. H3CI → H3C+ + I- slow stepstep 2. H3C+ + Cl- → H3CCl fast stepMechanism II: step 1. H3CI + Cl- → H3CClI slow stepstep 2. H3CClI → H3CCl + I- fast stepa.) Write a rate law for each reaction.Which of the following pairs of compounds reacts with OH- ions faster in an SN2 reaction? Briefly explain. CH3Br or CH3I? 2-chloro-2-methylpropane or chloromethane?

