Write the electron configurations for the following elements using spectroscopic (spdf) and orbital box notation. Identify the element as paramagnetic or diamagnetic. (a) Fe, iron Full electron configuration = (do not use noble gas notation) Orbital box notation: Зd 4s Зр 3s 2p 2s 1s Fe is (b) Se, selenium Noble gas electron configuration = Orbital box notation: 4p Se is
Write the electron configurations for the following elements using spectroscopic (spdf) and orbital box notation. Identify the element as paramagnetic or diamagnetic. (a) Fe, iron Full electron configuration = (do not use noble gas notation) Orbital box notation: Зd 4s Зр 3s 2p 2s 1s Fe is (b) Se, selenium Noble gas electron configuration = Orbital box notation: 4p Se is
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter7: The Structure Of Atoms And Periodic Trends
Section: Chapter Questions
Problem 49GQ: The magnet in the following photo is made from neodymium, iron, and boron. A magnet mode of on alloy...
Related questions
Question
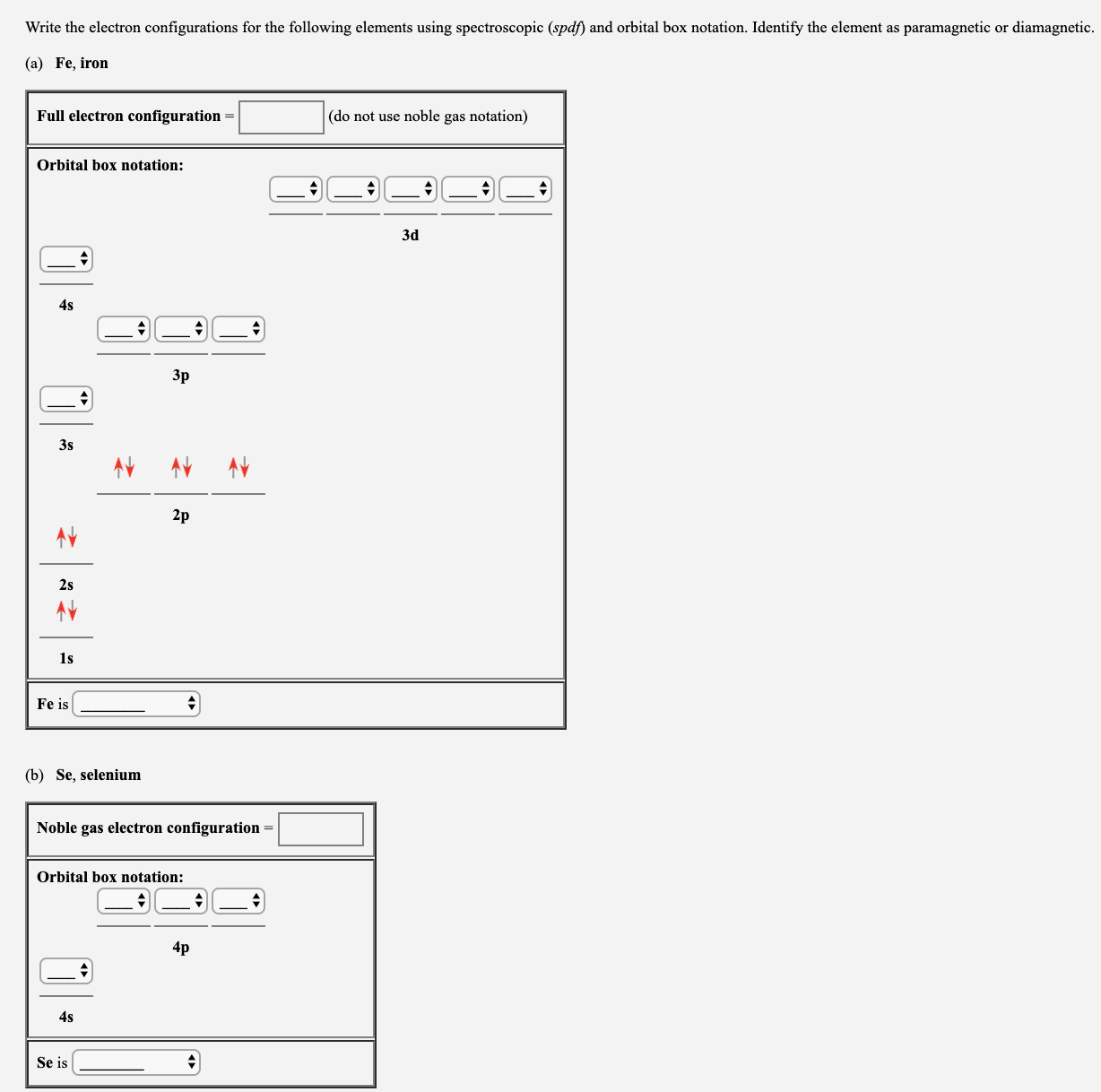
Transcribed Image Text:Write the electron configurations for the following elements using spectroscopic (spdf) and orbital box notation. Identify the element as paramagnetic or diamagnetic.
(a) Fe, iron
Full electron configuration =
(do not use noble gas notation)
Orbital box notation:
Зd
4s
Зр
3s
2p
2s
1s
Fe is
(b) Se, selenium
Noble gas electron configuration =
Orbital box notation:
4p
Se is
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning