You are given a mixture of CUCO, and CuO. You place the mixture in a crucible and heat it to decompose the CUCO; into CO, gas and CuO. You made sure that the CUCO, had completely decomposed by constantly heating it, cooling it, and weighing it until the mass remains the same. Using the data below from the experiment, calculate the % CUCO, in the original sample. Mass of empty crucible: 22.107 g Mass of crucible + sample before heating: 23.152 g Mass of crucible + sample at constant mass: 22.845 g Please do not include units in your answer and use proper sig figs. Assume that all of the given values have 3 digits after the decimal (even if Canvas drops the zero at the end).
You are given a mixture of CUCO, and CuO. You place the mixture in a crucible and heat it to decompose the CUCO; into CO, gas and CuO. You made sure that the CUCO, had completely decomposed by constantly heating it, cooling it, and weighing it until the mass remains the same. Using the data below from the experiment, calculate the % CUCO, in the original sample. Mass of empty crucible: 22.107 g Mass of crucible + sample before heating: 23.152 g Mass of crucible + sample at constant mass: 22.845 g Please do not include units in your answer and use proper sig figs. Assume that all of the given values have 3 digits after the decimal (even if Canvas drops the zero at the end).
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter9: Chemical Quantities
Section: Chapter Questions
Problem 6A
Related questions
Question
100%
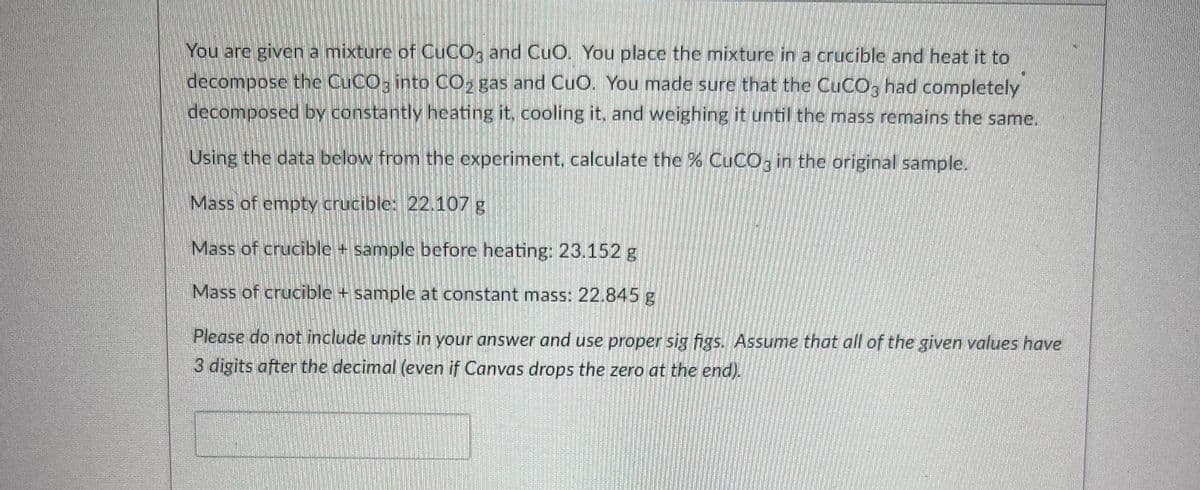
Transcribed Image Text:You are given a mixturc of CUCO, and CuO. You place the mixturcina crucible and heat it to
decompose the CUCO; into CO, gas and CuO. You made sure that the CUCO, had completely
decomposed by constantly heating it, cooling it, and weighing it until the mass remains the same.
Using the data below from the experiment, calculate the % CUCO; in the original sample.
Mass of empty crucible: 22.107 g
Mass of crucible + sample beforc heating: 23.152 g
Mass of crucible + sample at constant mass: 22.845g
Please do not include units in your answer and use proper sig figs. Assume that all of the given values have
3 digits after the decimal (even if Canvas drops the zero at the end).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning