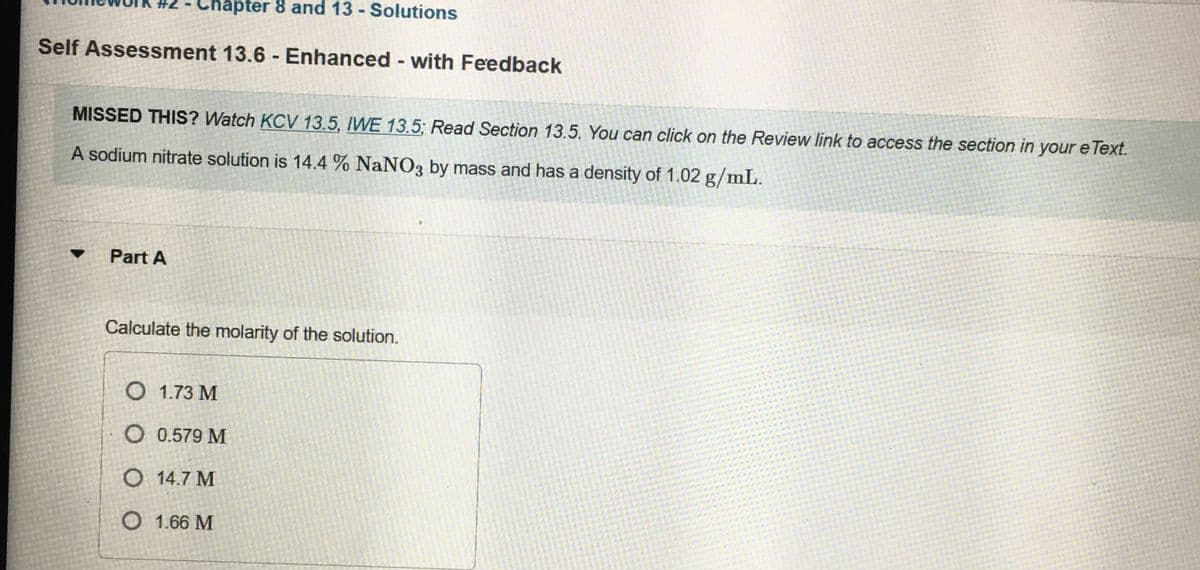
You can click on the Review link to access the se A sodium nitrate solution is 14.4 % NaNO3 by mass and has a density of 1.02 g/mL. Part A Calculate the molarity of the solution. O 1.73 M O 0.579 M O 14.7 M O 1.66 M
Q: Determine the pOH of a 0.00598 M HCIO4 solution. O 11.777 O 6.434 O 2.223 O 3.558 O 7.566
A: Given that, 0.00598 M of HClO4 solution. We have to calculate the pOH of the solution. So, the…
Q: Part E Find the percent dissociation of a 0.130 M solution of a weak monoprotic acid having Ka =…
A: Given : We have to calculate the percentage dissociation of weak acid.
Q: ball & stick labels cis isomer trans isomer cis-trans isomerism not possible
A:
Q: Which statement is true regarding drugs given intramuscularly? A. The drug goes directly to the…
A: Drugs can be given different ways- 1- orally it can be given 2- it can be given intramuscularly…
Q: Potassium dichromate is often used to standardize reducing agents like Fe2** Cr207 + 6Fe2+ + 14 H* →…
A: it is an example of redox titration where potassium dichromate is standardized using reducing agents…
Q: 1. Aqueous dilution of I0, results in the following reaction: 10. ja) + 2H,O = H,JO, jac) and K =…
A:
Q: Consider the 5 reactions shown below. 1. H2O(g) + CH3Brg) → CH3OH(g) + HBr(g) 2. 2 H2(g) + O2(g) → 2…
A: one mole of the substance is formed by its constituent elements in its standard states, the enthalpy…
Q: 18 of 21 I Review | Constants | Periodic Table The temperature for each solution is carried out at…
A: Given that: Mass of HCl = 0.80g Molar mass of HCl = 36.5g/mol Volume of solution = 3.5L
Q: salicylic acid point groups and symmetry detailed explanation
A: The point group of salicylic acid is
Q: 1. Aqueous dilution of I0, results in the following reaction: 10. jace) + 2H,O= HJO, jae) and K =…
A: GIVEN:-
Q: An electrochemical cell is constructed in which the silver ions in silver chloride are reduced to…
A: Given, A current of 0.500 A is passed through the cell for 101 minutes. The mass of copper metal…
Q: What product(s) forms at the anode in the electrolysis of an aqueous solution of FeBr2?
A: At anode oxidation takes place always.
Q: A solution prepared by mixing 22.2 mL of 0.400 M NaCl and 22.2 mL of 0.400 M KI was titrated with…
A: Answer is 7.48 x 10-16 M .
Q: What is the oxidation number of H in each of the following: H2(g); H2SO4; NaH For the toolbar, press…
A: Oxidation number Oxidation number of an atom/ion is the number of electrons an atom/ion that the…
Q: 2 N2H4(1) + N204(1) → 3 N2(g) + 4 H2O(g) Suppose we mix together 50 molecules of N2H4 and 45…
A: N2H4 reacts with N2O4 to form N2 and water. The equation for the balanced chemical reaction is as…
Q: PROCESS/PROCEDURE: Answer the questions below in relation to the following generic phase diagram.…
A: A question based on phase diagram that is to be accomplished.
Q: Compound A reacts with CrO, in dilute acid to give 2,3-dimethylbutanoic acid. What is compound A…
A: Organic reactions are those in which organic reactant react to form organic products.
Q: Write the full electron configuration for P-. Dreedback Start by determining the electron…
A: For neutral atom: Atomic number is equal to the number of electrons Electronic configuration:…
Q: A student titrates a water sample (V-100 ml) with 0.05N H2S04, find the quantity and quality of the…
A: A question based on concentration terms that is to be accomplished.
Q: Which of the following reactions will result in the formation of an acyl halide? Select one: a. The…
A:
Q: Several emergent properties of water contribute to the suitability of the environment for life. How…
A: The correct statements are : 2) The partial positive region of water molecules are attracted to…
Q: 12.) Which of the following is NOT true for the kinetic molecular theory A.) The volume occupied by…
A: * Kinetic energy of gas molecules is related to the motion of molecules . * Greater the…
Q: Draw the Newman and Sawhorse representation of ethane with the LOWEST POTENTIAL ENERGY.
A: Ethan has two different possible Newman and Sawhorse projections which are eclipsed and staggered.
Q: Part A A step-up transformer increases 19 V to 240 V What is the current in the secondary coil as…
A: We know that, power = voltage difference × electric current P = VI
Q: A chemist wishing to do an experiment requiring "Ca (half-life = 4.5 days) needs 6.0 ug of the…
A:
Q: 34. Which of the following statements about salicylic acid is true? A. has no solubility in the…
A:
Q: [Kererences) INTERACTIVE EXAMPLE Nuclear Binding Energy I Calculate the binding energy per nucleon…
A: Given:: mass of Fe = 55.9349 amu mass of proton = 1.0078 amu mass of neutron = 1.0087 amu
Q: A chemist wishing to do an experiment requiring "Ca²+ (half-life = 4.5 days) needs 5.0 µg of the…
A: Given, Half-life, t1/2 = 4.5 days Time, t = 96 h = 4 days Current quantity, N = 5 μg Original…
Q: Question 3 The biological systems of various marine life forms may be affected by the pH of the sea…
A: We are given the speciation diagram of H2CO3 that gives information about the fraction of each…
Q: Complete the following reactions: A. 2-pentene + water → B. cyclohexene + HCI - conc. H2 SO4 C. c.…
A: A] Product : 2-pentene Is a five carbon containing unsaturated hydrocarbon molecule,…
Q: 4. Potassium dichromate is often used to standardize reducing agents like Fe2+: Cr:0, + 6Fe +14 H*…
A: Given data,Volume of Fe2+=34.00mL=0.034LMass of K2Cr2O7=398.5mg=0.3985gMolar mass of…
Q: Calculate the expected absorbance value of a 0.39 M aqueous solution of a solution if the extinction…
A:
Q: The elements A and B combine to produce two different compounds: A3B and AB2. If 0.18 mol of A3B has…
A: We can calculate the molar mass of any substance by the following formula : Molar mass = mass of…
Q: b Bi undergoes four decay reactions: a, B, B, a. Step 1. 33 Bi undergoes a decay to give 31° TI In…
A:
Q: 14. In a voltaic cell, a zinc anode in a zinc sulfate solution is one half-cell, and a copper…
A: 14. A voltaic cell or galvanic cell is a device in which chemical energy is converted into…
Q: Calculate the pH of a 5.3 x 10-3-M solution of H2S04 (Ka, = 1.2 x 10-2). pH =
A: Given-> Concentration of H2SO4 = 5.3 × 10-3 M
Q: A 25.0-mt sample of an unknown H2CO3 solution requires 45.3 mL of 0.101 M NaOH for complete…
A: Given : Volume of H2CO3 solution = 25.0 ml Volume of NaOH solution = 45.3 ml Molar concentration…
Q: The decomposition of NO2(g) : 2NO2(g) ----->2NO(g) +O(g) is a second order reaction and the rate…
A:
Q: Data Table 1.513- Lay Mass of 4-nitrobenzaldehyde Mass of p-anisidine Mass of product Melting point…
A: A question based on concentration terms that is to be accomplished.
Q: Kelerences a. Be-7 decays by gamma emission. What are the atomic symbol and mass number of the…
A: The correct answer about radioactive decay is given below
Q: Which of the following is the balanced half -reaction for the oxidation of Cu to Cu+2? Select one:…
A: Oxidation :- The process of loss of electrons or increase in oxidation number is known as oxidation…
Q: a. At-209 decays by alpha decay. What are the atomic symbol and mass number of the product? Product:…
A: a. At -209 decays by alpha decay Answer - atomic symbol - Bi Mass number - 205…
Q: A 100.0-mL sample of spring water was treated to convert any iron present to Fe. Titration with…
A:
Q: Write the full electron configuration for P. St. full electron configuration: 12°2p°3s?sp TH or What…
A: Given-> P3- ->Negative charge means three electrons are added in phosphorus.
Q: What do you call the stage of the titration in which the number of moles of the titrant is…
A: In acid-base titration, two terms are very common - Titrant and Analyte Titrant : A solution,…
Q: For Cd(OH)2, Ksp = 5.9 x 10-15, For [Cd(CN)4]²- Kr = 3 × 1018. What is the molar solubility of…
A: Since you have asked multiple questions, we will solve the first question for you. If you want any…
Q: why do we need spindle cide in viscometer ?
A: By the help of Spindle (an immersed element) The Viscometer rotates a sensing element which…
Q: How much CO2 can be derived from 500 g lactose?
A: Chemical formula of lactose = C12H22O11 Molar mass of lactose = 342.3 g/mol
Q: b Bi undergoes four decay reactions: a, B, B, a. Step 1. 214 Bi undergoes a decay to give 20 TI 83…
A: Determine the symbol , mass number and atomic number of the given decay ---
Q: A titration is carried out where 50mL of 0.3M HF is titration with a 0.190M NaOH solution. Determine…
A:
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

- You are given the suggestion that a 0.15 ppm quinine aqueous solution is a good concentration to shoot for in the experiments, but quinine fluoresces best when it is acidified to 0.10 M H2SO4. Describe how to make 100 mL of the recommended solution starting with quinine sulfate (actually, quinine hemisulfate salt monohydrate). Assume you also have 1.0 M sulfuric acid and de-ionized water.Dilute the stock solution: Using the solutions in the burets, carefully measure the following quantities of Co(NO3)2 solution and water into 5 large test tubes, to a total of 5.00 mL in each test tube. Yes, the optional video says to make 10.00 mL total, but 5.00 mL is sufficient at this time and generates less waste. Use the buret to measure the cobalt solution and the water. Test tube No. 1 2 3 4 5 mL Co2+ Soln 1.00 2.00 3.00 4.00 5.00 mL H2O 4.00 3.00 2.00 1.00 0.00 Total mL 5.00 5.00 5.00 5.00 5.00 Mix each test tube thoroughly: hold the top of the tube and mix the bottom well. Transfer a portion of each diluted sample into a clean, dry sample cuvette. fill the concentration table and do calculations pleaseYou are supplied with the following: / Jy word voorsien van die volgende: NaCl(Mr= 58,443 g /mol) 2.5MTris-Cl, pH 8 solution /oplossing (1 Litre) EDTA,natriumsout(Mr= 380,2g/mol) 10% sodium dodecyl sulphate solution / natriumdodecyl sulfaat oplossing Proteïnase K solution / oplossing (50 mg dissolved / opgelos in 1 ml ddH2O) You need a digestion buffer consisting of the following: / Jy moet 'n verteringsbuffer op maak wat uit die volgende bestaan: 15m M NaCl 75 mM Tris-Cl,pH 8 16 mM EDTA,pH 8 0.8% sodium sulphate / natrium dodecyl sulfaat 0,75 mg/ml proteïnase K How will you prepare 500 ml of the digestion buffer? Show all your steps and calculations. Remember to explain exactly how you will make it up.
- Which statement is true? Statement 1: The two phases in the liquid/liquid extraction are aqueous layer and organic layerStatement 2: The distribution coefficient is the ratio of the equilibrium concentrations of the analyte in the two immiscible liquidsVolumetric Analysis The mass of KHC8H4O4 is measured to the nearest milligram; however, the volume of water in which it is dissolved is never of concern—water is even added to the wall of the Erlenmeyer flask during the titration. Explain why water added to the KHC8H4O4 has no effect on the data, whereas water added to the NaOH solution, may drastically affect the data.You are asked to make the compound [Ni(en)3]SO4. - By using 2.611 grams of NiSO4*6H2O (s) you dissolve the sample in deionized water completely. - You then add 10ml of 25% en into the solution and mix until a single product forms - When the addition of the en is complete, you then add 15ml of ethanol and mix. - using a bench vaccine and Buchner funnel, you filter the precipitate while carefully breaking up the solid until powder form. - the product is the washed with 15ml of ethanol and 15ml of acetone. Powder the solid and leave to dry Which is the limiting reagent? Write a balanced chemical equation for the reaction. How many miles of the product can you theoretically prepare?
- How much amount (in grams) do you need to prepare the following solutions 500.0 mL 0.1000 M stock EDTA solution from Na2H2EDTA•2H2O (FW=372.24) and MgCl2•6H2O crystals 100.0 mL 0.0500 M stock Ca2+ solution from pure CaCO3 (FW=100.09) and concentrated HCl 250 mL 1.0 M NH3-NH4+ pH 10 buffer solution from NH4Cl and NH3A 25.00 mL sample of an aqueous solution Y is extracted with 50.00 mL of hexane. After extraction, it was found that 94.12% of solute Y was successfully removed from the aqueous solution. Calculate: i. The distribution constant for this separation The initial concentration of Y if 1.77 mmol Y remains in aqueous solution after extraction. iii. The concentration of Y in BOTH phases after extractionPrepare 100.00 mL of a solution with ALL the following chemicals into together; CHEMICALS PROVIDED solid FeCl3 6H2O, iron(II) chloride hexahydrate (source of FeCl3) dilute hydrochloric acid in the rack – 3.0 M solid sodium salicylate – 99.7% assay Concentrations needed in the 100mL solution FeCl3: 0.020 M HCl: 0.050 M Salicylate (mg/L): 40.25 PLEASE SHOW HOW TO CALUATE AND WORK. The only equations you will need to use are the equations for molarity and dilutions.
- 5.00 mL of stock solution is diluted to 25.00 mL, producing solution ALPHA. 10.00 mL of solution ALPHA is diluted to 25.00 mL, resulting in solution BETA. 10.00 mL of solution BETA is then diluted to 25.00 mL, producing solution GAMMA. dilution factor for ALPHA from stock solution = 0.167 dilution factor for BETA from ALPHA solution = 0.0476 part c and d?Prepare 25ppm of Cd (NO3 )2.4H2O in a 500ml from 1000ppm stock solution of Cd (NO3)2.4H2OUse the attached pictures to help fill in the rest of the data. Please note that I used 0.79g of KMnO4 to prepare 250ml of 0.02M solution to titrate the ferrous ammonium sulfate. The approximate 1g of ferrous ammonium sulfate contained 25ml of distilled water and 10ml of 1M H2SO4 : H3PO4 to help dissolve the mixture for part one. For part two, the same steps were used however, we are to determin the amount of Fe in the sample. Please show and explain your work for each trail. Thank you!



