. The pyridine molecule (C3H;N) is obtained by replacing one C-H group in benzene with a nitrogen atom. Because nitrogen is more electronegative than the C-H group, orbitals with electron density on nitrogen are lower in energy. How do you expect the MOs and energy levels of pyridine to differ from those of benzene?
. The pyridine molecule (C3H;N) is obtained by replacing one C-H group in benzene with a nitrogen atom. Because nitrogen is more electronegative than the C-H group, orbitals with electron density on nitrogen are lower in energy. How do you expect the MOs and energy levels of pyridine to differ from those of benzene?
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter6: Quantum Mechanics And Molecular Structure
Section: Chapter Questions
Problem 63P: The pyridine molecule (C5H5N) is obtained by replacing one CH group in benzene with a nitrogen atom....
Related questions
Question
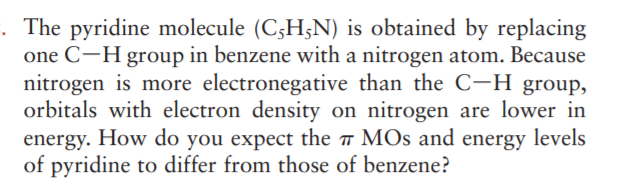
Transcribed Image Text:. The pyridine molecule (C3H;N) is obtained by replacing
one C-H group in benzene with a nitrogen atom. Because
nitrogen is more electronegative than the C-H group,
orbitals with electron density on nitrogen are lower in
energy. How do you expect the MOs and energy levels
of pyridine to differ from those of benzene?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning