. Which alkali metal would be expected to have the lowest first ionization energy? A/ . Which labeled element in the third period of the chart above has the highest quantity electron affinity energy? Type the letter from the given chart of the alkaline earth metal that has the largest atomic radius. A/ ● Select from the given letters, the letter of the element that would be the most electronegative on this periodic chart. A O II Activ Go to 63°F Cle
. Which alkali metal would be expected to have the lowest first ionization energy? A/ . Which labeled element in the third period of the chart above has the highest quantity electron affinity energy? Type the letter from the given chart of the alkaline earth metal that has the largest atomic radius. A/ ● Select from the given letters, the letter of the element that would be the most electronegative on this periodic chart. A O II Activ Go to 63°F Cle
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter9: Liquids, Solids, And Materials
Section9.9: Metals, Semiconductors, And Insulators
Problem 9.16CE: Look in Appendix D and compare the electron configurations shown there with the fusion enthalpies...
Related questions
Question
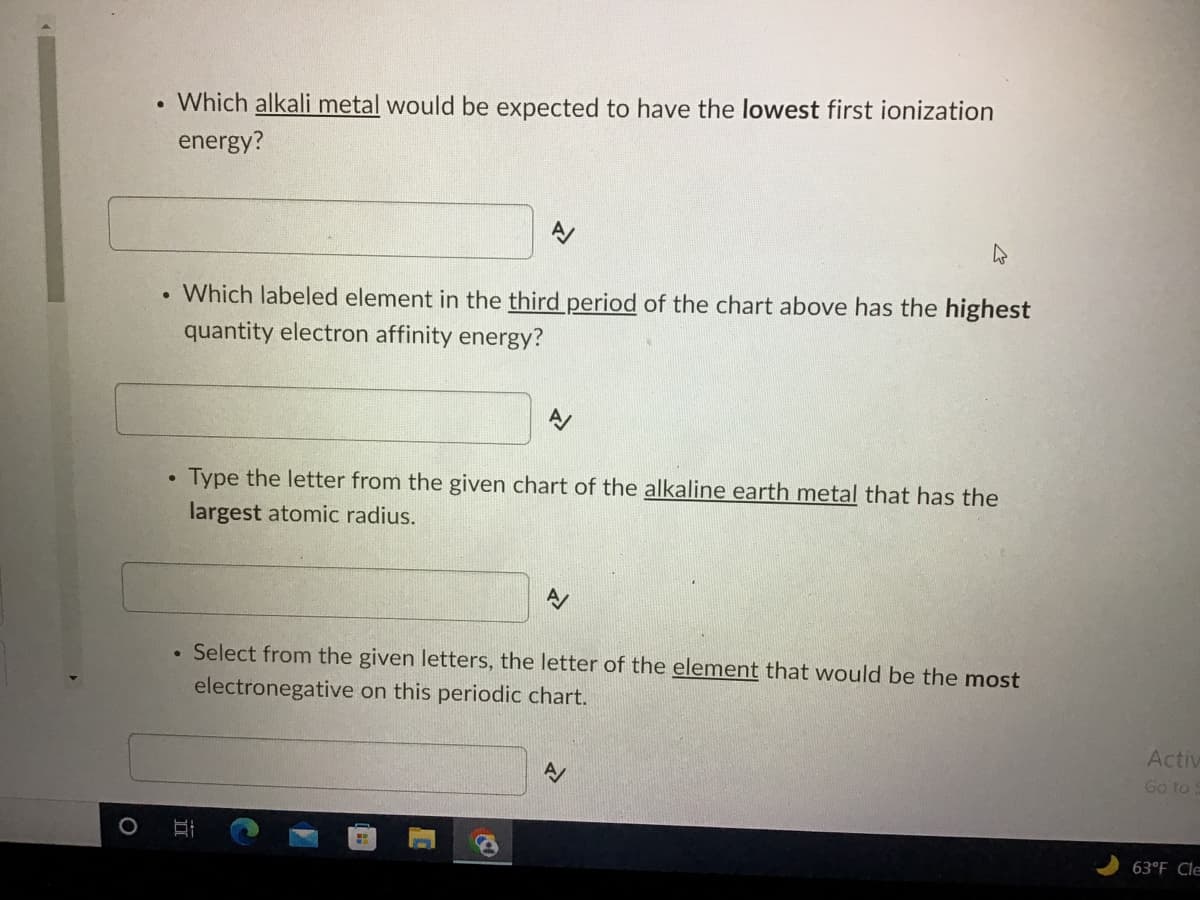
Transcribed Image Text:. Which alkali metal would be expected to have the lowest first ionization
energy?
W
●
Which labeled element in the third period of the chart above has the highest
quantity electron affinity energy?
A/
●
Type the letter from the given chart of the alkaline earth metal that has the
largest atomic radius.
A/
Select from the given letters, the letter of the element that would be the most
electronegative on this periodic chart.
A
O
II
Activ
Go to
63°F Cle
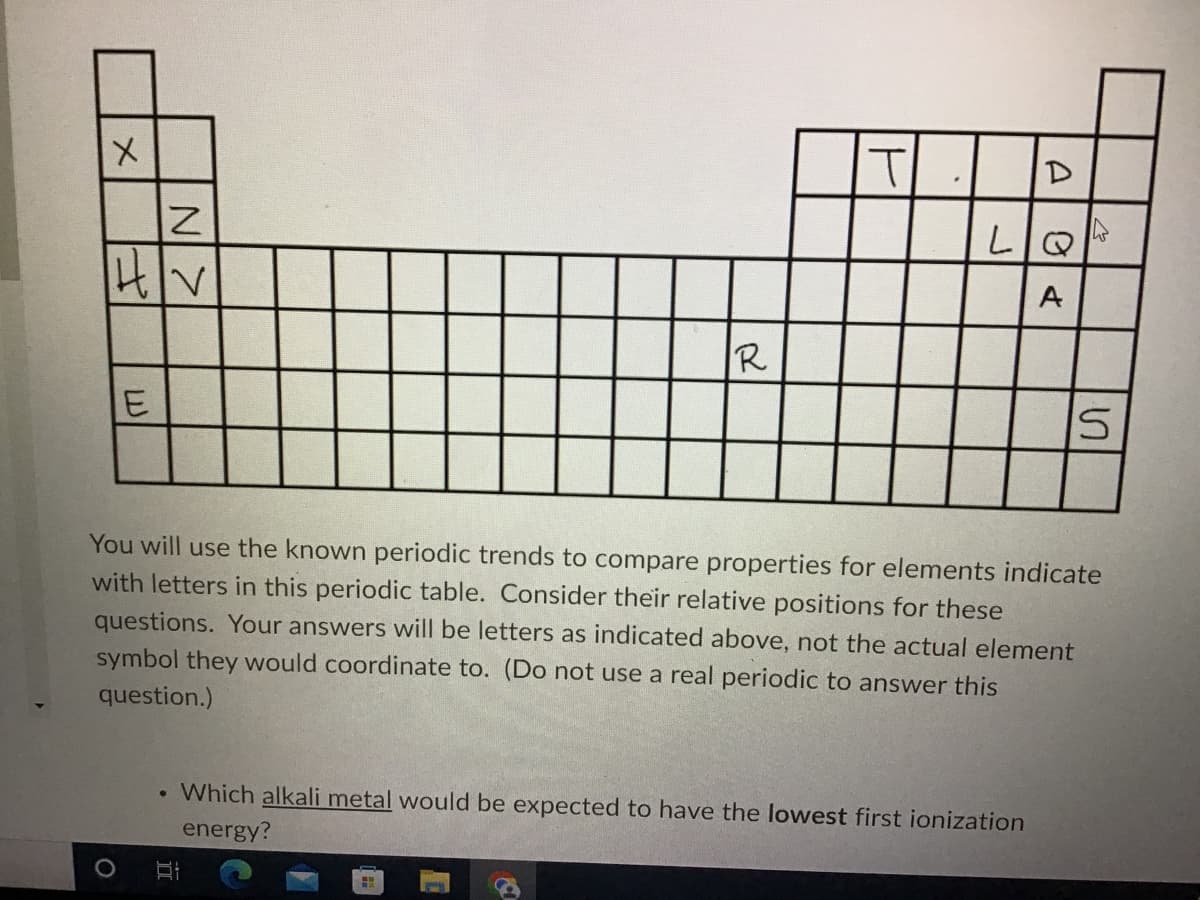
Transcribed Image Text:x
|H|V
R
E
S
You will use the known periodic trends to compare properties for elements indicate
with letters in this periodic table. Consider their relative positions for these
questions. Your answers will be letters as indicated above, not the actual element
symbol they would coordinate to. (Do not use a real periodic to answer this
question.)
●
Which alkali metal would be expected to have the lowest first ionization
energy?
BI
H
O
N>
T
D
LIQ
A
0
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning