إغلاق ...) (1501130 General chemistry 1 section2) 0.09 Hydrogen (H2), less dense than oxygen (02), .7 5: diffuses )1 نقطة( times as fast 1/16 times fast 16 times fast 4 times fast 1/4 CO(g) + 2H2(g) =CH3OH KP = 0.038.8 What is the value of Kc for the reaction at 200 C( %3D 5 R=0.0821 atm.L/mol.K)
إغلاق ...) (1501130 General chemistry 1 section2) 0.09 Hydrogen (H2), less dense than oxygen (02), .7 5: diffuses )1 نقطة( times as fast 1/16 times fast 16 times fast 4 times fast 1/4 CO(g) + 2H2(g) =CH3OH KP = 0.038.8 What is the value of Kc for the reaction at 200 C( %3D 5 R=0.0821 atm.L/mol.K)
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter16: Thermodynamics: Directionality Of Chemical Reactions
Section: Chapter Questions
Problem 122QRT
Related questions
Question
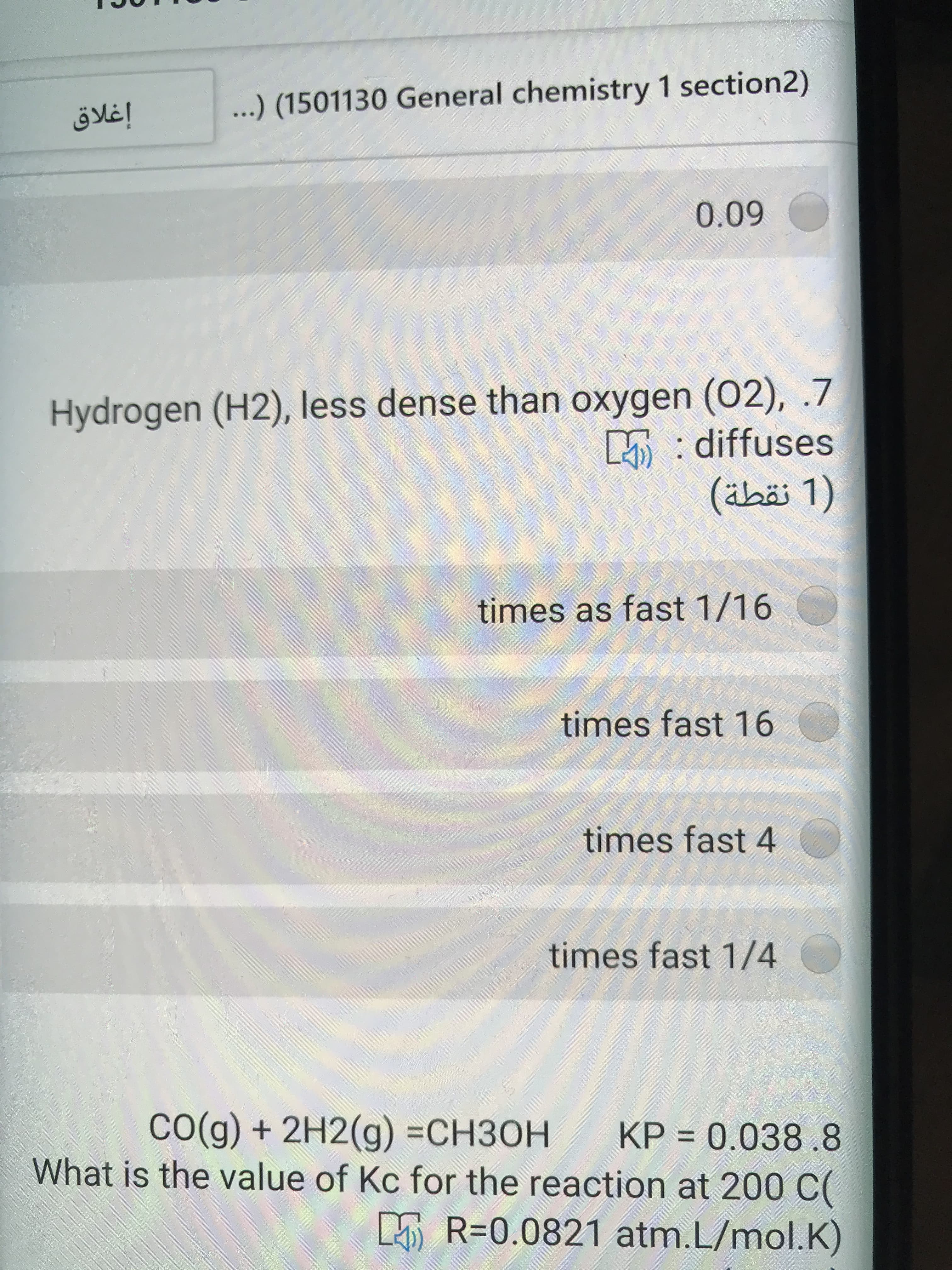
Transcribed Image Text:إغلاق
...) (1501130 General chemistry 1 section2)
0.09
Hydrogen (H2), less dense than oxygen (02), .7
5: diffuses
)1 نقطة(
times as fast 1/16
times fast 16
times fast 4
times fast 1/4
CO(g) + 2H2(g) =CH3OH KP = 0.038.8
What is the value of Kc for the reaction at 200 C(
%3D
5 R=0.0821 atm.L/mol.K)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning