What is Boyle's law constant, kp if a sealed rigid container with a volume, Vof 0.600 L has a pressure, Pof 130.0 kPa? V = kg(1/P) BIUA - A - I E E E X x, = E 12pt Paragraph fx MacBook Air
What is Boyle's law constant, kp if a sealed rigid container with a volume, Vof 0.600 L has a pressure, Pof 130.0 kPa? V = kg(1/P) BIUA - A - I E E E X x, = E 12pt Paragraph fx MacBook Air
Introductory Chemistry: A Foundation
8th Edition
ISBN:9781285199030
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter13: Gases
Section: Chapter Questions
Problem 137AP
Related questions
Question
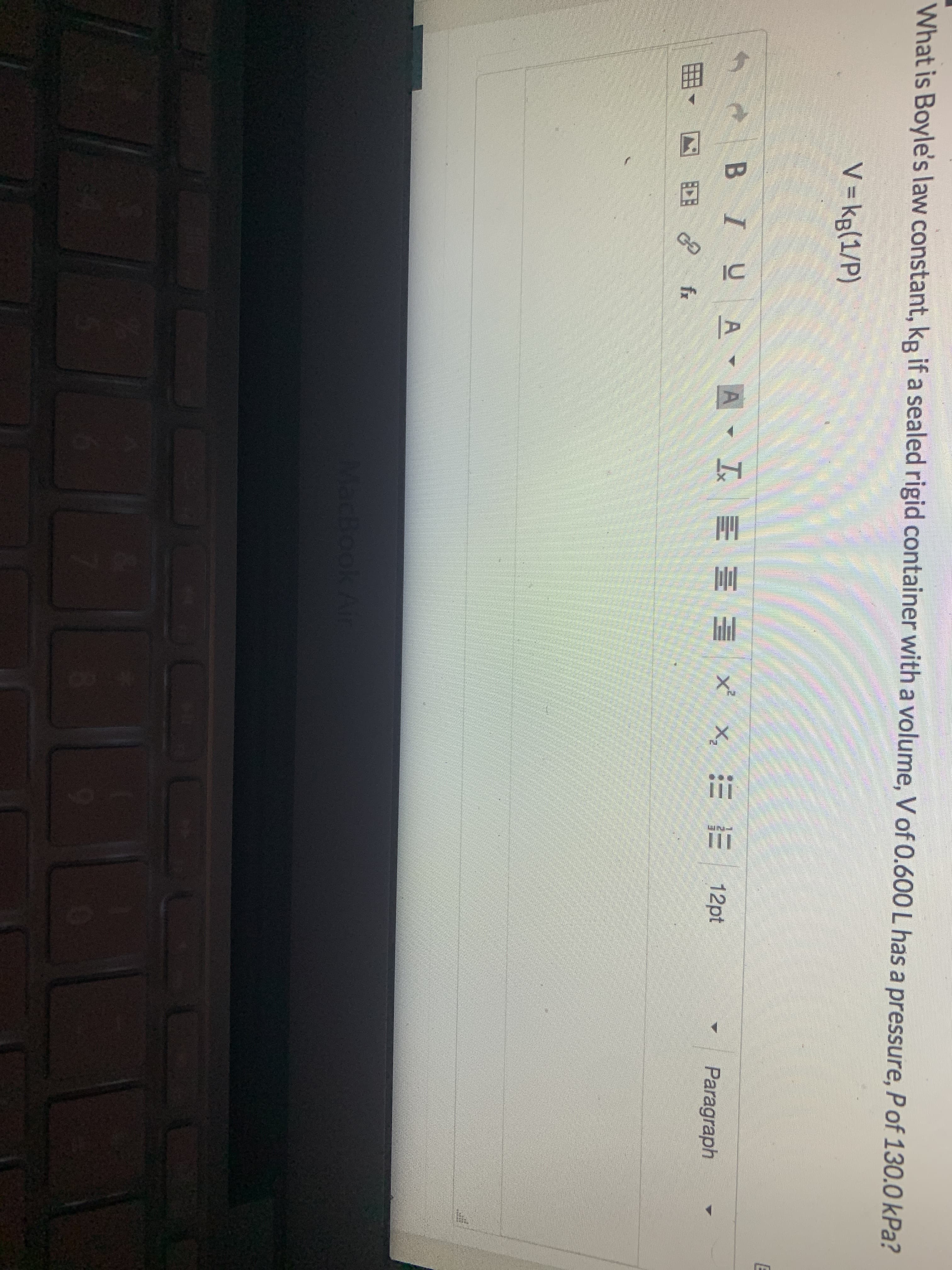
Transcribed Image Text:What is Boyle's law constant, kp if a sealed rigid container with a volume, Vof 0.600 L has a pressure, Pof 130.0 kPa?
V = kg(1/P)
BIUA - A - I E E E X x, = E 12pt
Paragraph
fx
MacBook Air
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning