... For each substance in the pair, list the forces between the formula units (IeFs for most, but IaFs for some!), compare their strength, and predict which has the higher m.p. or b.p: CH3 CH;NH, HB,DD,D CH;CH,NH, HB, DO,D* AICI; Lonic (LaFs) PCI, DD D CH;F DDD CH;NH, HB,DD,D Xe Li ( CH;OH HB.DD.D CO, DD,D SCI, SO2 LIF ÇH3 H3CC. H3C A2 HF Ha Hz CH3. CH3 H3C
... For each substance in the pair, list the forces between the formula units (IeFs for most, but IaFs for some!), compare their strength, and predict which has the higher m.p. or b.p: CH3 CH;NH, HB,DD,D CH;CH,NH, HB, DO,D* AICI; Lonic (LaFs) PCI, DD D CH;F DDD CH;NH, HB,DD,D Xe Li ( CH;OH HB.DD.D CO, DD,D SCI, SO2 LIF ÇH3 H3CC. H3C A2 HF Ha Hz CH3. CH3 H3C
Chapter4: Forces Between Particles
Section: Chapter Questions
Problem 4.78E
Related questions
Question
100%
I need help explaining the blanks
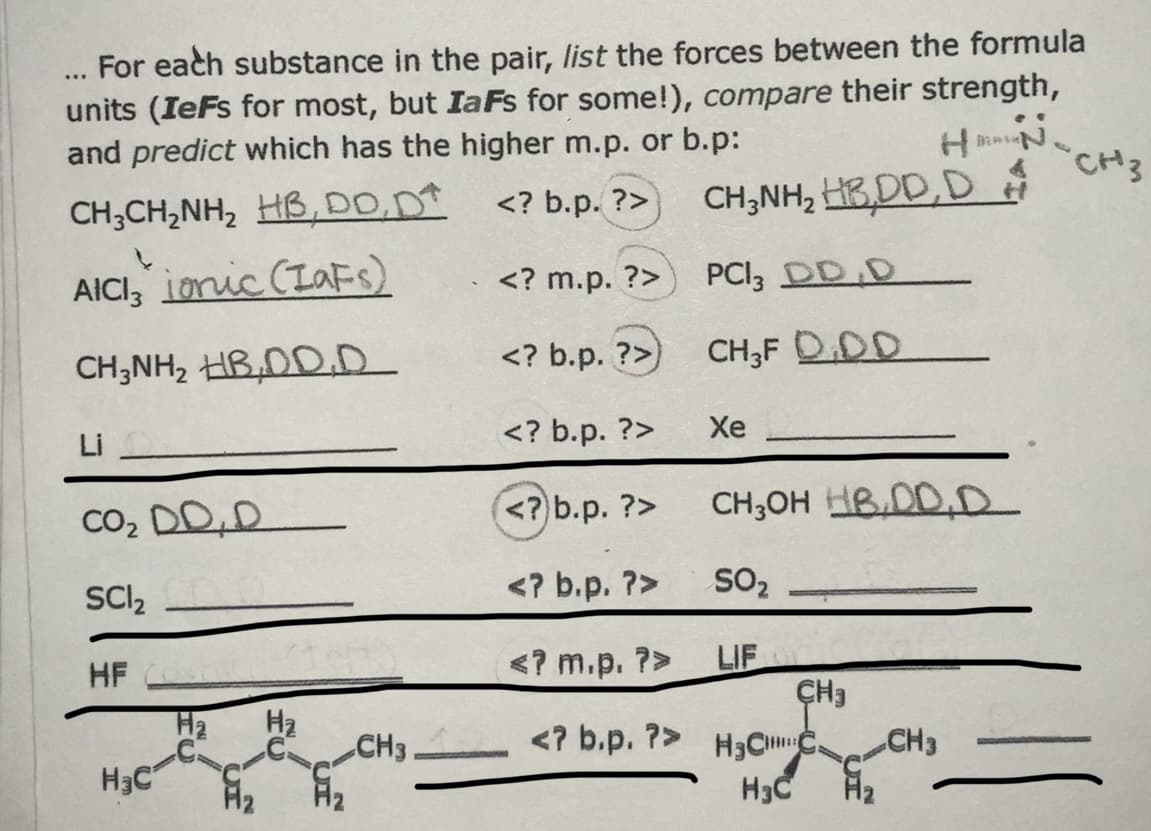
Transcribed Image Text:For each substance in the pair, list the forces between the formula
units (IeFs for most, but IaFs for some!), compare their strength,
and predict which has the higher m.p. or b.p:
...
CH;CH,NH, HB, DD,D <? b.p. ?>
CH3
CH;NH, HB,DD,D
AICI, Lonic (Lafs)
PCI; DD D
<? m.p. ?>
CH;NH, HB,DD.D
<? b.p. ?>)
CH;F D.DD
Li
<? b.p. ?>
Xe
Co, DD,D
(<?) b.p. ?>
CH;OH HBDD.D
SCI,
<? b.p. ?>
SO2
<? m.p. ?>
LIF
ÇH3
HF
H2
H2
CH3
<? b.p. ?>
H3CIC.
H3C
CH3
H3C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning