0.13 20 0.25 30 0,34 40 0.40 50 0.43 60 0.45 70 0.46 80 0.46 90 0.47 14. Use the following data to calculate the reaction rate law for the system: NO+ H HNO Experiment NO Initial Rate of (mol/L) (mol/L) Reaction (mol/(L's)) 0,001 0.004 0.002 2. 0.002 0.004 0.008 3. 0.003 0.004 0.018 0.004 0.001 0.008 0.004 0.002 0.016 6. 0.004 0.003 0.024 2 34
0.13 20 0.25 30 0,34 40 0.40 50 0.43 60 0.45 70 0.46 80 0.46 90 0.47 14. Use the following data to calculate the reaction rate law for the system: NO+ H HNO Experiment NO Initial Rate of (mol/L) (mol/L) Reaction (mol/(L's)) 0,001 0.004 0.002 2. 0.002 0.004 0.008 3. 0.003 0.004 0.018 0.004 0.001 0.008 0.004 0.002 0.016 6. 0.004 0.003 0.024 2 34
Chemistry: Matter and Change
1st Edition
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Chapter16: Reaction Rates
Section: Chapter Questions
Problem 6STP
Related questions
Question
The initial rate of the reaction: Br-(aq)+8H
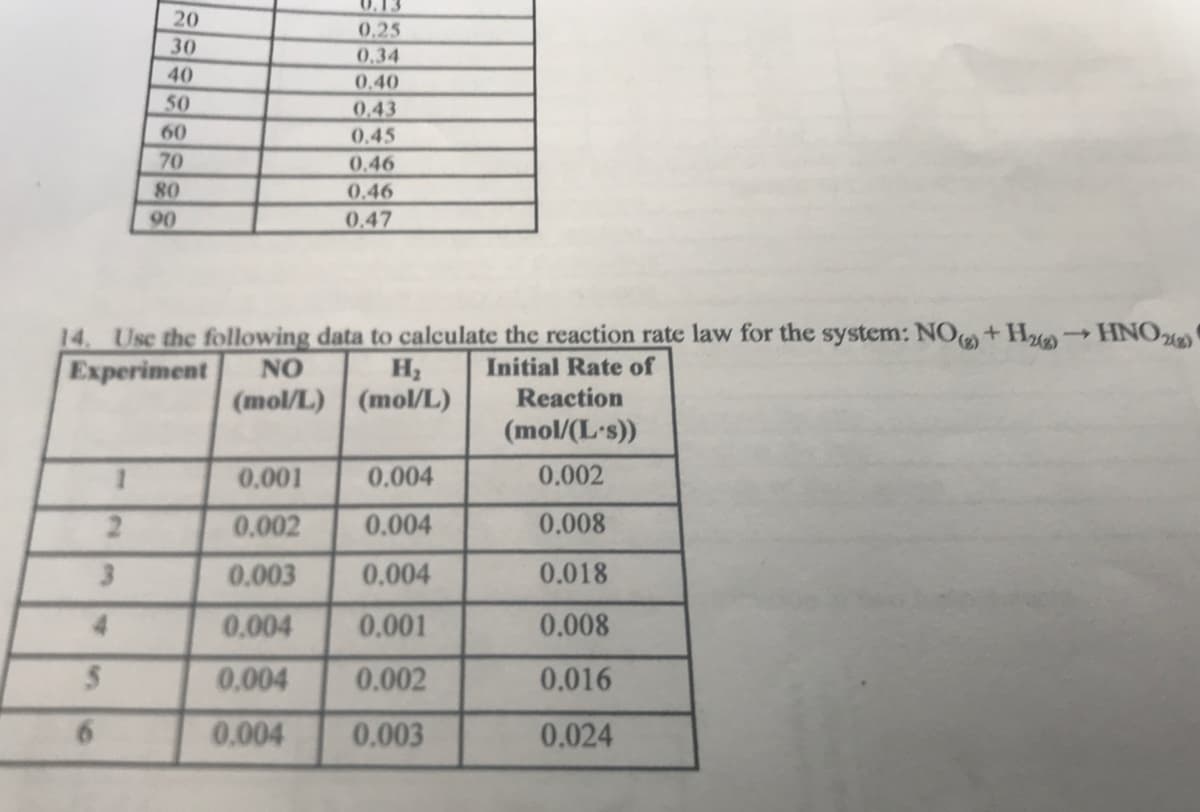
Transcribed Image Text:0.13
20
0,25
30
0,34
40
0.40
50
0.43
0.45
60
70
0.46
80
0.46
90
0.47
14. Use the following data to calculate the reaction rate law for the system: NO+ H HNO
Experiment
(3),
NO
Initial Rate of
(mol/L) (mol/L)
Reaction
(mol/(L's))
0.001
0.004
0.002
2.
0.002
0.004
0.008
3.
0.003
0.004
0.018
0,004
0.001
0.008
0.004
0.002
0.016
0.004
0.003
0.024
2 34
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning