0.2963 g of copper was deposited at the cathode after undergoing an electrolysis reaction. The electrodes were immersed in CuSO4 solution throughout the reaction. With the aid of Table Q2 (a), determine the amount of current flowing through the wire during the 10 minutes reaction. Table Q2 (a): Atomic Number and Atomic Mass of Elements Atomic Element Symbol Atomic number (2) Element Symbol Atomic number (2) Atomic mass (4) mass (4) Actinium Ac 89 227.0278 Mercury Hg 80 200.59 Aluminum Al 13 26.98154 Molybdenum Mo 42 95.94 Americium Am 95 Nd 60 144.24 Neodymium Neon Antimony Sb 51 121.75 Ne 10 20.179 Argon Ar 18 39.948 Neptunium Np 93 237.0482 Arsenic As 74.9216 Nickel Ni 28 58.7 Astatine At 85 -210 Niobium Nb 41 92.9064 Barium Ba 56 137.33 Nitrogen N 7 14.0067 Berkelium Bk 97 ~247 Nobelium No 102 -259 Beryllium Be 4 9.01218 Osmium Os 76 190.2 Bismuth Bi 83 208.9804 Oxygen 0 8 15.9994 Boron B 5 10.81 Palladium Pd 46 106.4 Bromine Br 35 79.904 Phosphorus P 15 30.97376 Cd 48: 112.41 Platinum Pt 78 195.09 Cadmium Calcium Californium Ca 20 40.08 Plutonium Pu -244 CF 98 Polonium Po 84 -209 251 12.011 C K 19 39.0983 Carbon Cerium Potassium Praseodymium 140.12 59 140.9077 Ce Cs CI 132.9054 Promethium Pm 61 -145 35.453 Protactinium Pa 231.0359 Cesium Chlorine Chromium Cobalt Cr 51.996 Radium Ra 88 226.0254 Co 58.9332 Radon Rn 86 -222 Cu 63.546 Rhenium Re 75 186.207 Cm Rhodium Rh 45 102.9055 Rubidium ~247 162.5 -254 Rb 37 Copper Curium Dysprosium Einsteinium Erbium Europium 85.4678 Dy Es Ruthenium Ru 44 101.07 167.26 Samarium Sm 150.4 Eu 151.96 Scandium Se 21 44.9559 Fermium Fm Se 34 78.96 -257 18.998403 Fluorine F Si 14 28.0855 Fr 47 107.868 -223 157.25 Gd 11 22.98977 Ga 69.72 38 87.62 Francium Gadolinium Gallium Germanium Gold Hafnium 16 32.06 72.59 196.9665 Au Selenium Silicon Silver Sodium Strontium Sulfur Tantalum Technetium Tellurium Terbium Thallium Thorium Thulium 73 180.9479 Hf He 178.49 4.0026 164.9304 52 127.6 158.9254 Ho 65 Helium Holmium Hydrogen Indium lodine H 1.0079 81 204.37 232.0381 In 114,82 90 T 126.9045 69 168.9342 Ir 192.22 50 118.69 Fe 55.847 22 47.9 Iridium Iron Krypton Lanthanum Tin Titanium Tungsten Uranium Kr 74 183.85 83.8 138.9055 238.029 Lr -260 23 50.9414 Lawrencium Lead Pb 207.2 54 6.941 173.04 Vanadium Xenon Ytterbium Yttrium Zine Zirconium Lu 174.97 39 Lithium Lutetium Magnesium Manganese 88.9059 Mg 24.305 30 65.38 Mn 54.938 40 91.22 **52-362883338-13-22NNG-23EAST E-H 24 27 100 72 77 71 25 225-2992FEF>> Ag Na Ta Te Te Th Tm V Xe Yb Y 28
0.2963 g of copper was deposited at the cathode after undergoing an electrolysis reaction. The electrodes were immersed in CuSO4 solution throughout the reaction. With the aid of Table Q2 (a), determine the amount of current flowing through the wire during the 10 minutes reaction. Table Q2 (a): Atomic Number and Atomic Mass of Elements Atomic Element Symbol Atomic number (2) Element Symbol Atomic number (2) Atomic mass (4) mass (4) Actinium Ac 89 227.0278 Mercury Hg 80 200.59 Aluminum Al 13 26.98154 Molybdenum Mo 42 95.94 Americium Am 95 Nd 60 144.24 Neodymium Neon Antimony Sb 51 121.75 Ne 10 20.179 Argon Ar 18 39.948 Neptunium Np 93 237.0482 Arsenic As 74.9216 Nickel Ni 28 58.7 Astatine At 85 -210 Niobium Nb 41 92.9064 Barium Ba 56 137.33 Nitrogen N 7 14.0067 Berkelium Bk 97 ~247 Nobelium No 102 -259 Beryllium Be 4 9.01218 Osmium Os 76 190.2 Bismuth Bi 83 208.9804 Oxygen 0 8 15.9994 Boron B 5 10.81 Palladium Pd 46 106.4 Bromine Br 35 79.904 Phosphorus P 15 30.97376 Cd 48: 112.41 Platinum Pt 78 195.09 Cadmium Calcium Californium Ca 20 40.08 Plutonium Pu -244 CF 98 Polonium Po 84 -209 251 12.011 C K 19 39.0983 Carbon Cerium Potassium Praseodymium 140.12 59 140.9077 Ce Cs CI 132.9054 Promethium Pm 61 -145 35.453 Protactinium Pa 231.0359 Cesium Chlorine Chromium Cobalt Cr 51.996 Radium Ra 88 226.0254 Co 58.9332 Radon Rn 86 -222 Cu 63.546 Rhenium Re 75 186.207 Cm Rhodium Rh 45 102.9055 Rubidium ~247 162.5 -254 Rb 37 Copper Curium Dysprosium Einsteinium Erbium Europium 85.4678 Dy Es Ruthenium Ru 44 101.07 167.26 Samarium Sm 150.4 Eu 151.96 Scandium Se 21 44.9559 Fermium Fm Se 34 78.96 -257 18.998403 Fluorine F Si 14 28.0855 Fr 47 107.868 -223 157.25 Gd 11 22.98977 Ga 69.72 38 87.62 Francium Gadolinium Gallium Germanium Gold Hafnium 16 32.06 72.59 196.9665 Au Selenium Silicon Silver Sodium Strontium Sulfur Tantalum Technetium Tellurium Terbium Thallium Thorium Thulium 73 180.9479 Hf He 178.49 4.0026 164.9304 52 127.6 158.9254 Ho 65 Helium Holmium Hydrogen Indium lodine H 1.0079 81 204.37 232.0381 In 114,82 90 T 126.9045 69 168.9342 Ir 192.22 50 118.69 Fe 55.847 22 47.9 Iridium Iron Krypton Lanthanum Tin Titanium Tungsten Uranium Kr 74 183.85 83.8 138.9055 238.029 Lr -260 23 50.9414 Lawrencium Lead Pb 207.2 54 6.941 173.04 Vanadium Xenon Ytterbium Yttrium Zine Zirconium Lu 174.97 39 Lithium Lutetium Magnesium Manganese 88.9059 Mg 24.305 30 65.38 Mn 54.938 40 91.22 **52-362883338-13-22NNG-23EAST E-H 24 27 100 72 77 71 25 225-2992FEF>> Ag Na Ta Te Te Th Tm V Xe Yb Y 28
Chapter18: Electrochemistry
Section: Chapter Questions
Problem 115AE: The saturated calomel electrode. abbreviated SCE. is often used as a reference electrode in making...
Related questions
Question
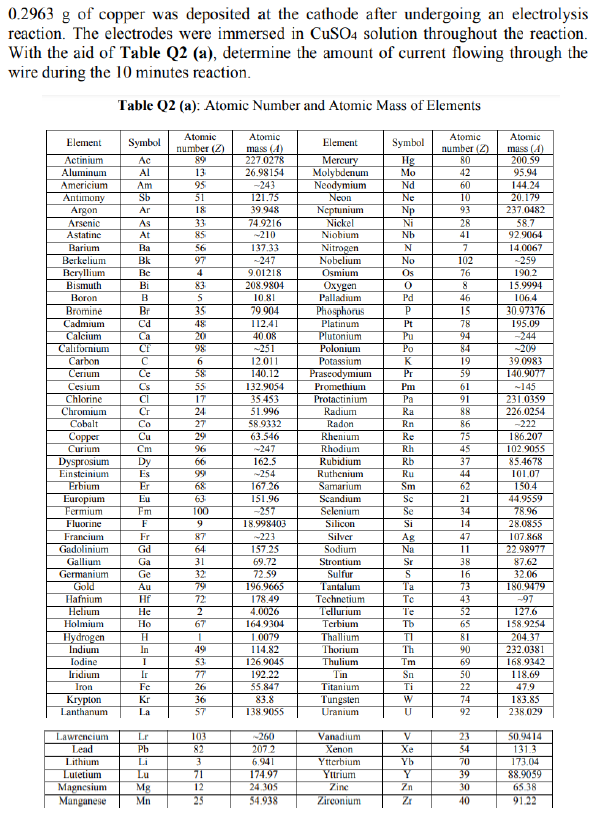
Transcribed Image Text:0.2963 g of copper was deposited at the cathode after undergoing an electrolysis
reaction. The electrodes were immersed in CuSO4 solution throughout the reaction.
With the aid of Table Q2 (a), determine the amount of current flowing through the
wire during the 10 minutes reaction.
Table Q2 (a): Atomic Number and Atomic Mass of Elements
Atomic
Atomic
Element
Symbol
Element
Symbol
Atomic
number (2)
Atomic
mass (4)
number (Z)
mass (4)
Actinium
Ac
89
227.0278
Mercury
Hg
80
200.59
Aluminum
Al
13
26.98154
Molybdenum
Mo
42
95.94
Americium
Am
95
-243
Nd
60
144.24
Neodymium
Neon
Antimony
Sb
51
121.75
Ne
10
20.179
Argon
Ar
18
39.948
Neptunium
Np
93
237.0482
Arsenic
As
33
74.9216
Nickel
Ni
28
58.7
Astatine
At
85
-210
Niobium
Nb
41
92.9064
Barium
Ba
56
137.33
Nitrogen
N
7
14.0067
Berkelium
Bk
97
-247
Nobelium
No
102
-259
Beryllium
Be
4
9.01218
Osmium
Os
76
190.2
Bismuth
Bi
83
208.9804
Oxygen
0
8
15.9994
Boron
B
5
10.81
Palladium
Pd
46
106.4
Bromine
Br
35
79.904
Phosphorus
P
15
30.97376
Cadmium
Cd
48
112,41
Platinum
Pt
78
195.09
Calcium
Ca
20
40.08
Plutonium
Pu
94
-244
Californium
CE
98
-251
Polonium
Po
84
-209
Carbon
C
6
12.011
Potassium
K
19
39.0983
Cerium
Ce
58
140.12
Praseodymium
Pr
59
140.9077
Cesium
Cs
55
132.9054
Promethium
Pm
61
-145
Chlorine
CI
17
35.453
Protactinium
Pa
91
231.0359
Chromium
Cr
24
51.996
Radium
Ra
88
226.0254
Cobalt
Co
27
58.9332
Radon
Rn
86
-222
29
63.546
Rhenium
Re
75
186.207
96
-247
Rhodium
Rh
45
102.9055
Copper
Curium
Dysprosium
Einsteinium
Erbium
66
162.5
Rubidium
Rb
37
85.4678
99
Ruthenium
Ru
44
101.07
-254
167.26
68
Samarium
Sm
62
150.4
Scandium
Sc
21
44.9559
Europium
Fermium
151.96
-257
18.998403
Selenium
Se
34
78.96
Fluorine
Silicon
Si
14
28.0855
Francium
-223
Silver
Ag
47
107.868
157.25
Sodium
Na
11
22.98977
69.72
Strontium
38
87.62
Gadolinium
Gallium
Germanium
Gold
72.59
Sulfur
16
32.06
196.9665
Tantalum
73
180.9479
Technetium
43
-97
Hafnium
Helium
178.49
4.0026
Tellurium
52
127.6
Holmium
164.9304
Terbium
65
158.9254
1.0079
Thallium
81
204.37
Hydrogen
Indium
Iodine
114.82
Thorium
90
232.0381
126.9045
Thulium
69
168.9342
192.22
Tin
50
118.69
Iridium
Iron
Krypton
55.847
Titanium
22
47.9
83.8
Tungsten
74
183.85
Lanthanum
138.9055
Uranium
92
238.029
Lawrencium
-260
Vanadium
50.9414
Lead
207.2
Xenon
54
131.3
Lithium
6.941
Ytterbium
70
173.04
Lutetium
Lu
174.97
Yttrium
39
88.9059
Magnesium Mg
24.305
30
65.38
Zine
Zirconium
Manganese
Mn
54.938
40
91.22
5864435
Cu
Cm
Dy
Er
Fm
F
Fr
+3682222==
Gd
Ga
Ge
Au
Hf
He
Ho
H
In
I
Ir
Fe
Kr
La
Lr
Pb
五
188888:
100
9
87
64
31
32
72
2
67
1
49
53.
77
26
36
57
103
82
3
71
12
25
Sr
S
Ta
Te
Te
Tb
TI
Th
Tm
Sn
Ti
W
U
V
Xe
Yb
Y
Zn
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning