1 Answer this! Directions: Use the periodic table you created on the previous slide to complete these questions. 1. Identify the halogen in the 3rd period ? Identify the alkaline earth metal in the 4th period ? Identify a metalloid with four valence electrons 3. ? What element is group with the metals but is really a nonmetal? ?_ Identify a noble gas in the 5th period ? Identify the alkali metal with the least amount of protons 7. ? Identify the transition metal in the 4th period and 7th group 8. ? Identify the element that does not have the same number of valence electrons as the others in its group 9. ? What element is group with the metalloid but is really a metal? 10. ? Identify an element that exists as a liquid under standard conditions 2. 4. 5. 6.
1 Answer this! Directions: Use the periodic table you created on the previous slide to complete these questions. 1. Identify the halogen in the 3rd period ? Identify the alkaline earth metal in the 4th period ? Identify a metalloid with four valence electrons 3. ? What element is group with the metals but is really a nonmetal? ?_ Identify a noble gas in the 5th period ? Identify the alkali metal with the least amount of protons 7. ? Identify the transition metal in the 4th period and 7th group 8. ? Identify the element that does not have the same number of valence electrons as the others in its group 9. ? What element is group with the metalloid but is really a metal? 10. ? Identify an element that exists as a liquid under standard conditions 2. 4. 5. 6.
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter2: Atoms Molecules And Ions
Section: Chapter Questions
Problem 103GQ
Related questions
Question
100%
please, help and answer the questions, thankuu! ?.
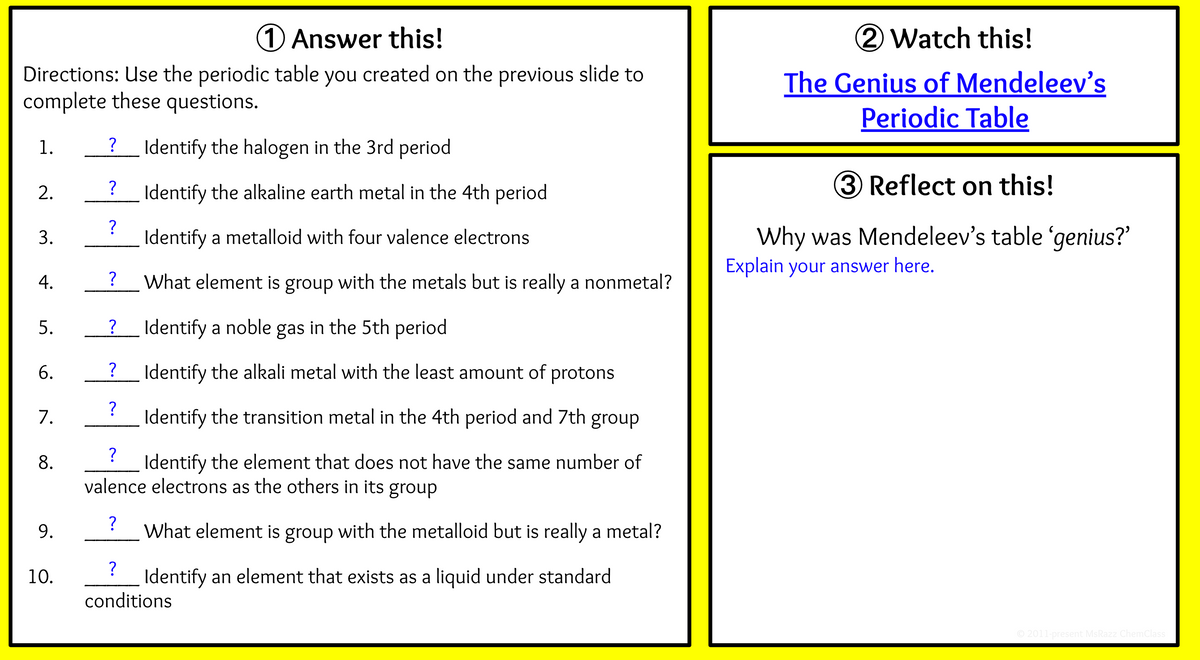
Transcribed Image Text:1Answer this!
2 Watch this!
Directions: Use the periodic table you created on the previous slide to
complete these questions.
The Genius of Mendeleev's
Periodic Table
1.
?
Identify the halogen in the 3rd period
?
Identify the alkaline earth metal in the 4th period
3 Reflect on this!
2.
?
Why was Mendeleev's table 'genius?
Explain your answer here.
Identify a metalloid with four valence electrons
?
What element is group with the metals but is really a nonmetal?
Identify a noble gas in the 5th period
?
Identify the alkali metal with the least amount of protons
?
7.
Identify the transition metal in the 4th period and 7th group
?
8.
Identify the element that does not have the same number of
valence electrons as the others in its group
9.
?
What element is group with the metalloid but is really a metal?
?
10.
Identify an element that exists as a liquid under standard
conditions
© 2011-present MsRazz ChemClass
3.
4.
5.
6.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning