1) Determine the total volume of the standards by using a formula in the appropriate boxes (your instructor will help you with this). 2) Determine the concentration of Cu2+ in each standard, also using a formula in the appropriate cells. 3) Plot the absorbance as a function of Cu2+ concentration. Determine the equation for the best-fit line.
1) Determine the total volume of the standards by using a formula in the appropriate boxes (your instructor will help you with this). 2) Determine the concentration of Cu2+ in each standard, also using a formula in the appropriate cells. 3) Plot the absorbance as a function of Cu2+ concentration. Determine the equation for the best-fit line.
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
ChapterA1: Evaluation Of Analytical Data
Section: Chapter Questions
Problem A1.23QAP
Related questions
Question
Can I please get help on steps 1-3
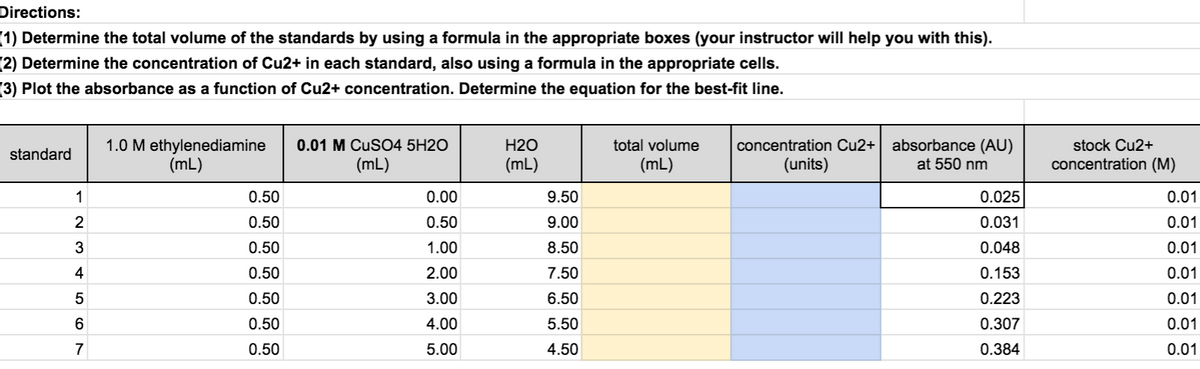
Transcribed Image Text:Directions:
(1) Determine the total volume of the standards by using a formula in the appropriate boxes (your instructor will help you with this).
(2) Determine the concentration of Cu2+ in each standard, also using a formula in the appropriate cells.
(3) Plot the absorbance as a function of Cu2+ concentration. Determine the equation for the best-fit line.
1.0 M ethylenediamine
(mL)
concentration Cu2+ absorbance (AU)
at 550 nm
0.01 M CuSO4 5H2O
H2O
total volume
stock Cu2+
standard
(mL)
(mL)
(mL)
(units)
concentration (M)
1
0.50
0.00
9.50
0.025
0.01
2
0.50
0.50
9.00
0.031
0.01
3
0.50
1.00
8.50
0.048
0.01
4
0.50
2.00
7.50
0.153
0.01
5
0.50
3.00
6.50
0.223
0.01
0.50
4.00
5.50
0.307
0.01
7
0.50
5.00
4.50
0.384
0.01
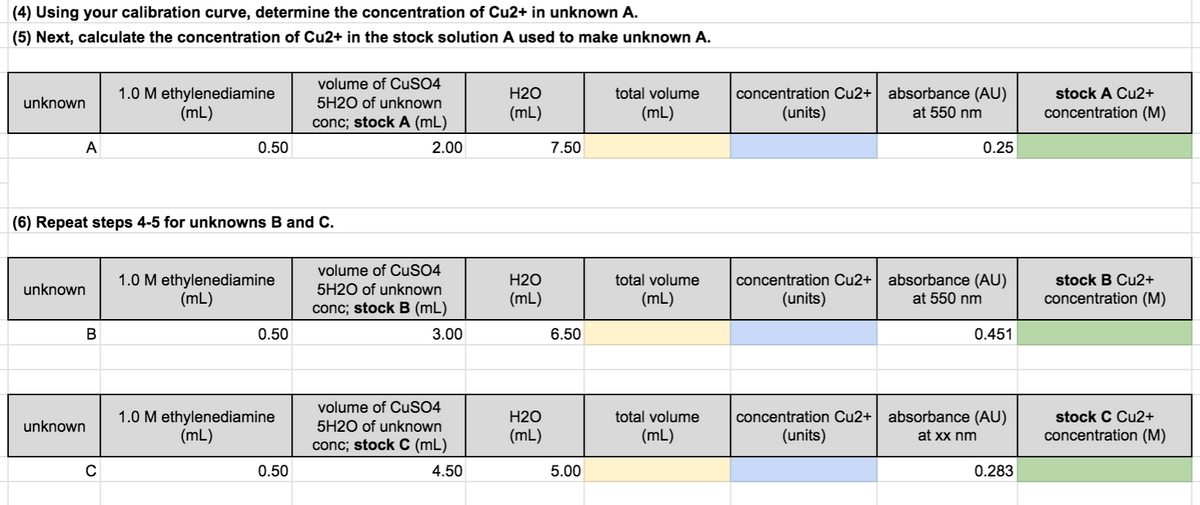
Transcribed Image Text:(4) Using your calibration curve, determine the concentration of Cu2+ in unknown A.
(5) Next, calculate the concentration of Cu2+ in the stock solution A used to make unknown A.
volume of CUSO4
absorbance (AU)
1.0 M ethylenediamine
(mL)
H2O
total volume
concentration Cu2+
stock A Cu2+
unknown
5H20 of unknown
(mL)
(mL)
(units)
at 550 nm
concentration (M)
conc; stock A (mL)
A
0.50
2.00
7.50
0.25
(6) Repeat steps 4-5 for unknowns B and C.
volume of CuSO4
1.0 M ethylenediamine
(mL)
total volume
(mL)
concentration Cu2+ absorbance (AU)
at 550 nm
H2O
stock B Cu2+
unknown
5H2O of unknown
(mL)
(units)
concentration (M)
conc; stock B (mL)
В
0.50
3.00
6.50
0.451
volume of CuSO4
concentration Cu2+| absorbance (AU)
1.0 M ethylenediamine
(mL)
H2O
total volume
stock C Cu2+
unknown
5H2O of unknown
(mL)
(mL)
(units)
at xx nm
concentration (M)
conc; stock C (mL)
0.50
4.50
5.00
0.283
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
