Nitrite ion, NO2-, is a preservative for bacon and other foods, but it is potentially carcinogenic. A spectrophotometric determination of NO2- makes use of the following reactions: A 5.00 cm cell was used to measure absorbance. Here is an abbreviated procedure for the determination. 1. To 50.0 mL of unknown solution containing nitrite is added 1.00 mL of sulfanilic acid solution. 2. After 10 min, 2.00 mL of 1-aminonapthalene solution and 1.00 mL of buffer are added. 3. After 15 min, the absorbance is read at 520 nm in a 5.00-cm cell. The following solutions were annaylzed: A. 50.0 mL of food extract known to contain no nitrite(that is a neglible amount); final absorbance =0.153 B. 50.0 mL of food extract suspected of containg nitrite ; final absorbance= 0.622 C. Same as B, but with 10 microliters of 7.50x10^-3 M NaNO2 added to the 50.0 mL sample; final absorbance= 0.967 a) Calculate the molar absorptivity of the colored product. Remember that a 5.00 cm cell was used b) How many micrograms of NO2- were present in 50.0 mL of food extract?
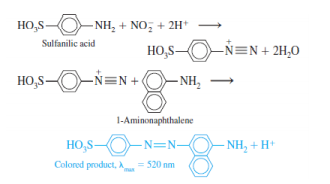
Nitrite ion, NO2-, is a preservative for bacon and other foods, but it is potentially carcinogenic. A spectrophotometric determination of NO2- makes use of the following reactions:
A 5.00 cm cell was used to measure absorbance. Here is an abbreviated procedure for the determination.
1. To 50.0 mL of unknown solution containing nitrite is added 1.00 mL of sulfanilic acid solution.
2. After 10 min, 2.00 mL of 1-aminonapthalene solution and 1.00 mL of buffer are added.
3. After 15 min, the absorbance is read at 520 nm in a 5.00-cm cell.
The following solutions were annaylzed:
A. 50.0 mL of food extract known to contain no nitrite(that is a neglible amount); final absorbance =0.153
B. 50.0 mL of food extract suspected of containg nitrite ; final absorbance= 0.622
C. Same as B, but with 10 microliters of 7.50x10^-3 M NaNO2 added to the 50.0 mL sample; final absorbance= 0.967
a) Calculate the molar absorptivity of the colored product. Remember that a 5.00 cm cell was used
b) How many micrograms of NO2- were present in 50.0 mL of food extract?
a) The absorbance due to the coloured product from nitrite added to sample C is,
0.967 - 0.622 = 0.345.
The concentration of coloured product due to added nitrite sample in C is,
Trending now
This is a popular solution!
Step by step
Solved in 2 steps







