1) Volume of liquid Liquid 1 Liquid 2 Type of liquid Water unknown liquid Volume (mL) 10.0 mL 10.0 mL 2) Mass of liquid Mass of dry beaker (or a graduated cylinder) 29.05 29.219 Mass of beaker (graduated cylinder) + liquid 38.789 37,00g 7.795 Mass of liquid 9.73g Density of the liquid (use Table 2 in your lab manual) Type of the liquid (use Table 2 in your lab manual)
1) Volume of liquid Liquid 1 Liquid 2 Type of liquid Water unknown liquid Volume (mL) 10.0 mL 10.0 mL 2) Mass of liquid Mass of dry beaker (or a graduated cylinder) 29.05 29.219 Mass of beaker (graduated cylinder) + liquid 38.789 37,00g 7.795 Mass of liquid 9.73g Density of the liquid (use Table 2 in your lab manual) Type of the liquid (use Table 2 in your lab manual)
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter1: Matter And Measurements
Section: Chapter Questions
Problem 73QAP: A Different civilization on a distant planet has developed a new temperature scale based on ethyl...
Related questions
Question
I am having trouble understanding the density of the liquid and type of liquid

Transcribed Image Text:B. Density of a liquid
1) Volume of liquid
Liquid 1
Liquid 2
Type of liquid
Water
unknown liquid
Volume (mL)
10.0 mL
10.0 ml
2) Mass of liquid
Mass of dry beaker (or a graduated cylinder) 29.05q
29.219
Mass of beaker (graduated cylinder) + liquid 38.789 3 l,00g
ור .ר
9.73g
Mass of liquid
Density of the liquid (use Table 2 in your lab manual)
Type of the liquid (use Table 2 in your lab manual)
sic
C. Desity of solid by direct measurement
r biloa lol
1) Mass of a solid (metal cylinder)
18.30g
(Jm) biloe touley
2) Diameter of the metal cylinder
5.1am
Height of the metal cylinder
bilos to
4,8441 235 cm?
3) Volume (in Cm³) of the metal cylinder ( V = n d? h/4)
4) Density of the metal cylinder
Ty d?h
7 1.1 5.1/4
니
3.7994
20
LABORATORY 2 | DENSITY OF SOLIDS & LIQUIDS: POST-LAB
POST-LAB
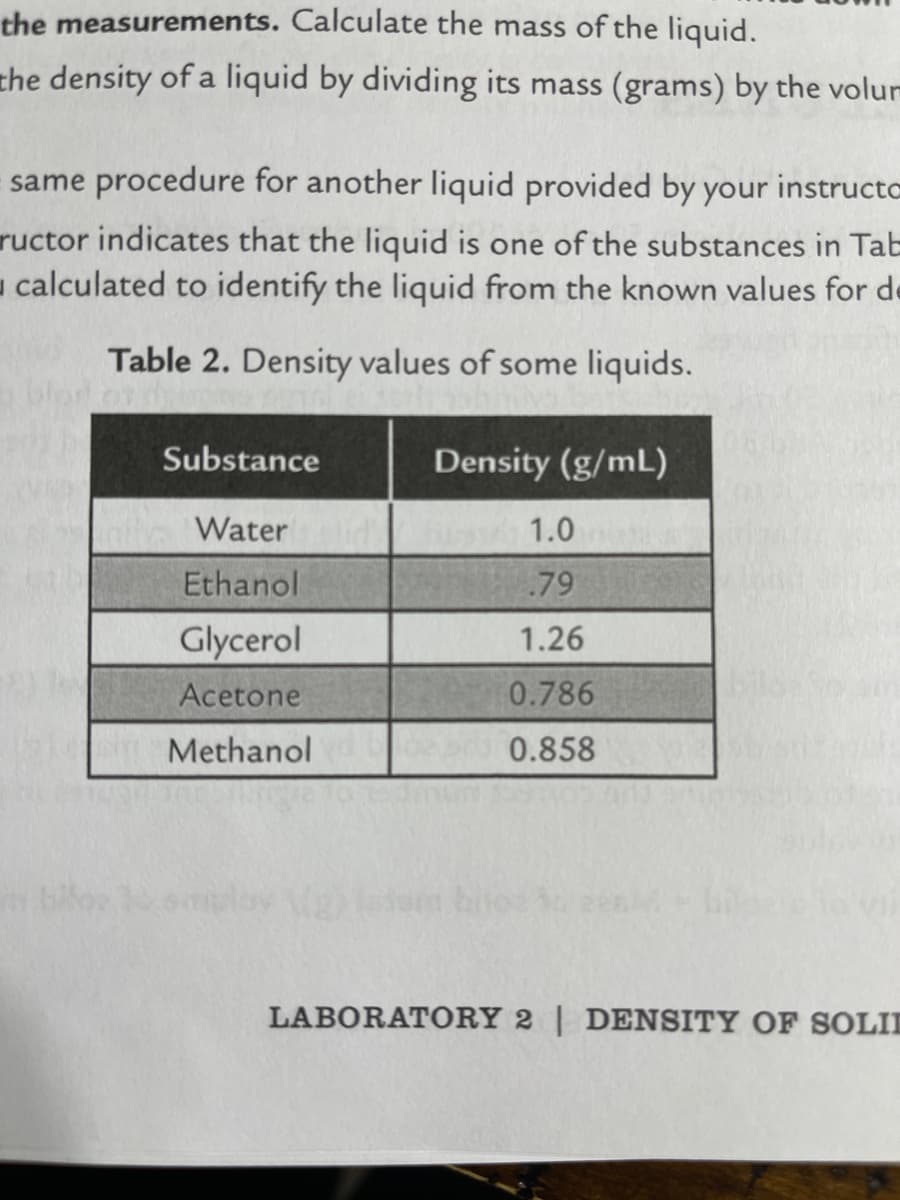
Transcribed Image Text:the measurements. Calculate the mass of the liquid.
the density of a liquid by dividing its mass (grams) by the volur
same procedure for another liquid provided by your instructo
ructor indicates that the liquid is one of the substances in Tab
- calculated to identify the liquid from the known values for de
Table 2. Density values of some liquids.
Substance
Density (g/mL)
Water
1.0
Ethanol
.79
Glycerol
1.26
Acetone
0.786
Methanol
0.858
am bitos o e
LABORATORY 2 | DENSITY OF SOLID
Expert Solution
Step 1
The mass and volume of liquid data given are,

Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning