1. Answer the following questions based on the table: Pickling Vinegar Conc NaOH (M) 0.1005 Trial #1 Trial #2 Trail #3 Trial #4 Volume Vinegar (mL) 2.00 2.00 2.00 2.00 Initial Volume NaOH (mL) 0.32mL 0.22 1.02 0.44 Final Volume (mL) 24.25mL 24.20 25.00 24.45 Volume NaOH added (mL) 23.93mL 23.98mL 23.98mL 24.01mL a) In the titration of vinegar, a student uses 12.36 mL of NaOH(aq) (Molarity 0.100 M) to titrate a 2.00 mL sample of vinegar. If the student used lithium hydroxide (Molarity: 0.200 M) instead, what volume of lithium hydroxide would you expect them to use? Show balanced equations. b) A student performs titrations of equivalent amounts of HNO, (aq) with 0.1 M NaOH(aq). The volumes of NaOH(aq) needed to titrate are measured to be: 18.09 mL, 17.92 mL, 18.17 mL. Using % difference, does the student need to perform another titration or are their values within acceptable agreement?
1. Answer the following questions based on the table: Pickling Vinegar Conc NaOH (M) 0.1005 Trial #1 Trial #2 Trail #3 Trial #4 Volume Vinegar (mL) 2.00 2.00 2.00 2.00 Initial Volume NaOH (mL) 0.32mL 0.22 1.02 0.44 Final Volume (mL) 24.25mL 24.20 25.00 24.45 Volume NaOH added (mL) 23.93mL 23.98mL 23.98mL 24.01mL a) In the titration of vinegar, a student uses 12.36 mL of NaOH(aq) (Molarity 0.100 M) to titrate a 2.00 mL sample of vinegar. If the student used lithium hydroxide (Molarity: 0.200 M) instead, what volume of lithium hydroxide would you expect them to use? Show balanced equations. b) A student performs titrations of equivalent amounts of HNO, (aq) with 0.1 M NaOH(aq). The volumes of NaOH(aq) needed to titrate are measured to be: 18.09 mL, 17.92 mL, 18.17 mL. Using % difference, does the student need to perform another titration or are their values within acceptable agreement?
Basic Clinical Lab Competencies for Respiratory Care: An Integrated Approach
5th Edition
ISBN:9781285244662
Author:White
Publisher:White
Chapter13: Oxygen Administration
Section: Chapter Questions
Problem 10SEPT
Related questions
Question
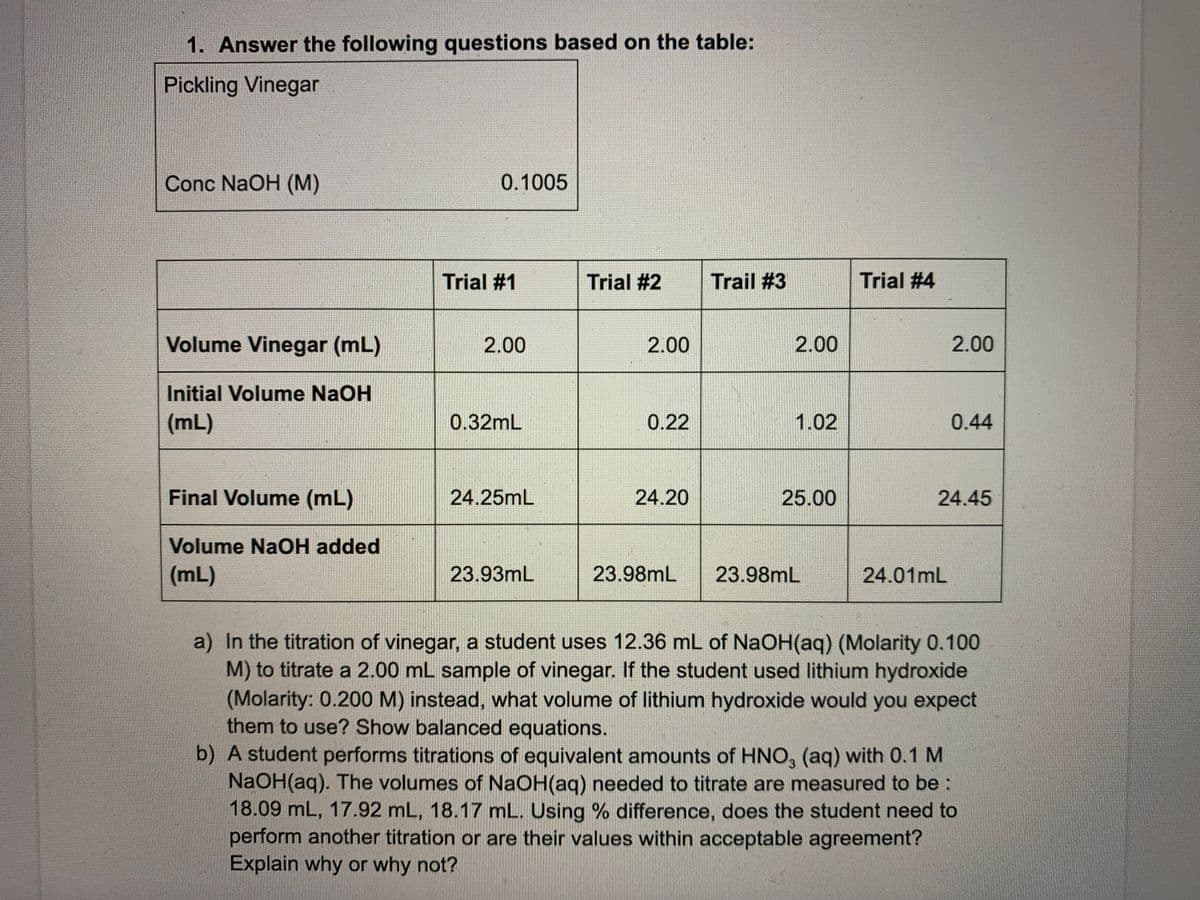
Transcribed Image Text:1. Answer the following questions based on the table:
Pickling Vinegar
Conc NaOH (M)
0.1005
Trial #1
Trial #2
Trail #3
Trial #4
Volume Vinegar (mL)
2.00
2.00
2.00
2.00
Initial Volume NaOH
(mL)
0.32mL
0.22
1.02
0.44
Final Volume (mL)
24.25mL
24.20
25.00
24.45
Volume NaOH added
(mL)
23.93mL
23.98mL
23.98mL
24.01mL
a) In the titration of vinegar, a student uses 12.36 mL of NaOH(aq) (Molarity 0.100
M) to titrate a 2.00 mL sample of vinegar. If the student used lithium hydroxide
(Molarity: 0.200 M) instead, what volume of lithium hydroxide would you expect
them to use? Show balanced equations.
b) A student performs titrations of equivalent amounts of HNO, (aq) with 0.1 M
NaOH(aq). The volumes of NaOH(aq) needed to titrate are measured to be:
18.09 mL, 17.92 mL, 18.17 mL. Using % difference, does the student need to
perform another titration or are their values within acceptable agreement?
Explain why or why not?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Basic Clinical Lab Competencies for Respiratory C…
Nursing
ISBN:
9781285244662
Author:
White
Publisher:
Cengage


Cardiopulmonary Anatomy & Physiology
Biology
ISBN:
9781337794909
Author:
Des Jardins, Terry.
Publisher:
Cengage Learning,

Basic Clinical Lab Competencies for Respiratory C…
Nursing
ISBN:
9781285244662
Author:
White
Publisher:
Cengage


Cardiopulmonary Anatomy & Physiology
Biology
ISBN:
9781337794909
Author:
Des Jardins, Terry.
Publisher:
Cengage Learning,
