1. ASPARTAME, CHN,Os, is an artificial sweetener that is 160 times sweeter than sucrose (sugar or table sugar) when dissolved in water. It is marketed as Nutra-Sweet. Calculate: A. molecular weight (MW) of the compound B. no. of molecules in 5.0 g aspartame C. mass in gram of 2.25 mols aspartame of aspartame D. atoms of C present grams of N present grams of 2.0 x 10° molecules E. F. As an agriculturist, which of the following compounds, (NH.),SO., KNO, and Ca(NO,)2, will you recommend for your farmers as source of nitrogen. Assume cost per kilogram (Kg) is the same. (Support answer with computations). 3. Calculate the density in g/L for nitrogen dioxide, NO, at STP and at 100 °C and five times the standard pressure.(3 points) 4. Four grams of methane, CH, (MW = 16 g/mol), at 27.0 °C and a pressure of 2.50 atm occupies a volume of 2.46 L. Calculate the value of the gas constant, R, in mL-atm / °C-mol. 2.
1. ASPARTAME, CHN,Os, is an artificial sweetener that is 160 times sweeter than sucrose (sugar or table sugar) when dissolved in water. It is marketed as Nutra-Sweet. Calculate: A. molecular weight (MW) of the compound B. no. of molecules in 5.0 g aspartame C. mass in gram of 2.25 mols aspartame of aspartame D. atoms of C present grams of N present grams of 2.0 x 10° molecules E. F. As an agriculturist, which of the following compounds, (NH.),SO., KNO, and Ca(NO,)2, will you recommend for your farmers as source of nitrogen. Assume cost per kilogram (Kg) is the same. (Support answer with computations). 3. Calculate the density in g/L for nitrogen dioxide, NO, at STP and at 100 °C and five times the standard pressure.(3 points) 4. Four grams of methane, CH, (MW = 16 g/mol), at 27.0 °C and a pressure of 2.50 atm occupies a volume of 2.46 L. Calculate the value of the gas constant, R, in mL-atm / °C-mol. 2.
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter8: Chemical Composition
Section: Chapter Questions
Problem 128CP: itamin B12 , cyancobalamin, is essential for human nutrition. Its molecular formula is...
Related questions
Question
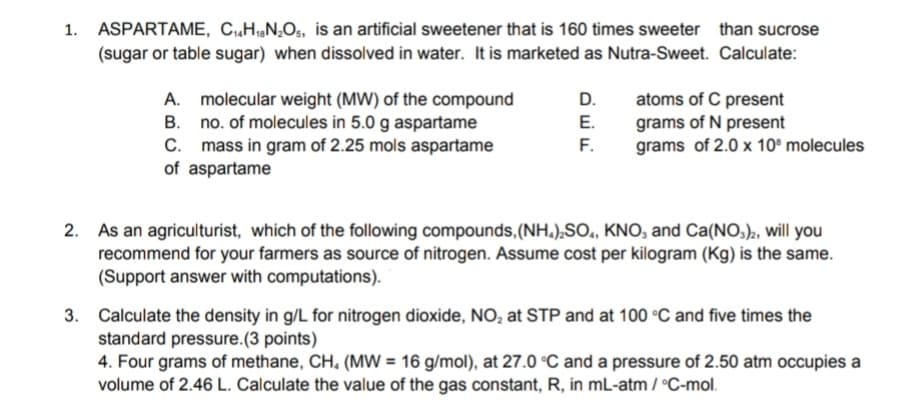
Transcribed Image Text:1. ASPARTAME, CH,N,O,, is an artificial sweetener that is 160 times sweeter than sucrose
(sugar or table sugar) when dissolved in water. It is marketed as Nutra-Sweet. Calculate:
A. molecular weight (MW) of the compound
B. no. of molecules in 5.0 g aspartame
C. mass in gram of 2.25 mols aspartame
of aspartame
D.
atoms of C present
grams of N present
grams of 2.0 x 10° molecules
E.
F.
As an agriculturist, which of the following compounds,(NH.),SO., KNO, and Ca(NO,)2, will you
recommend for your farmers as source of nitrogen. Assume cost per kilogram (Kg) is the same.
(Support answer with computations).
3. Calculate the density in g/L for nitrogen dioxide, NO, at STP and at 100 °C and five times the
standard pressure.(3 points)
4. Four grams of methane, CH, (MW = 16 g/mol), at 27.0 °C and a pressure of 2.50 atm occupies a
volume of 2.46 L. Calculate the value of the gas constant, R, in mL-atm / °C-mol.
2.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning