1. Calculate [H+], [OH-], pH and pOH of:
a. 0.1 M HNO3
b. 2.00 M (weak acid) HF (Ka=6.8 x 10^-4)
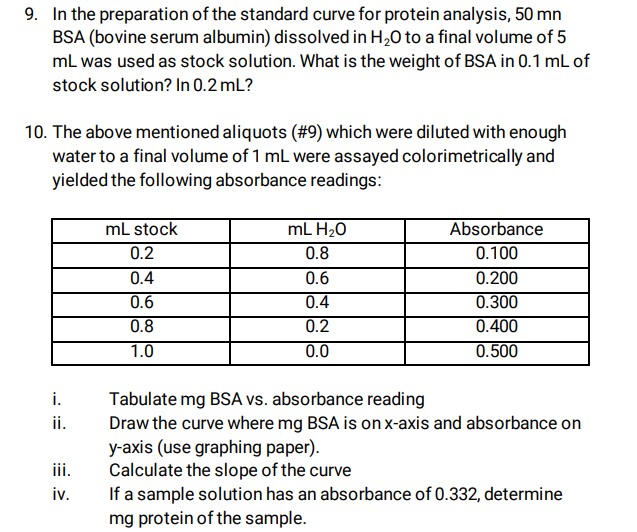
2. In the preparation of the standard curve for protein analysis, 50 mn
BSA (bovine serum albumin) dissolved in H2O to a final volume of 5
mL was used as stock solution. What is the weight of BSA in 0.1 mL of
stock solution? In 0.2 mL?
3. . The above mentioned aliquots (#9) which were diluted with enough
water to a final volume of 1 mL were assayed colorimetrically and
yielded the following absorbance readings:
mL stock mL H2O Absorbance
0.2 0.8 0.100
0.4 0.6 0.200
0.6 0.4 0.300
0.8 0.2 0.400
1.0 0.0 0.500
i. Tabulate mg BSA vs. absorbance reading
ii. Draw the curve where mg BSA is on x-axis and absorbance on
y-axis (use graphing paper).
iii. Calculate the slope of the curve
iv. If a sample solution has an absorbance of 0.332, determine
mg protein of the sample.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images







