1. Calculate the entropy change in (J/K-mol) for the process: H2O (L, 1.6 atm) ? H2O (G, 0.3 atm). The standard molar enthalpy of vaporization is 40.7 kJ/mole. ANS in 0 decimal place
1. Calculate the entropy change in (J/K-mol) for the process: H2O (L, 1.6 atm) ? H2O (G, 0.3 atm). The standard molar enthalpy of vaporization is 40.7 kJ/mole. ANS in 0 decimal place
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter10: Gases And Their Properties
Section: Chapter Questions
Problem 55PS: Consider a 5.00-L tank containing 325 g of H2O at a temperature of 275 C. (a) Calculate the pressure...
Related questions
Question
PLEASE ANSWER I ONLY HAVE 30 MINS LEFT! I'LL GIVE THUMBS UP!
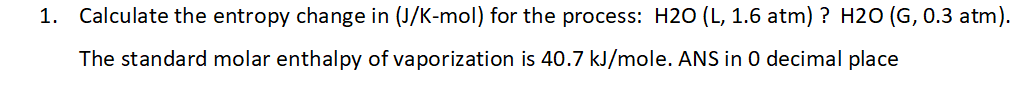
Transcribed Image Text:1. Calculate the entropy change in (J/K-mol) for the process: H2O (L, 1.6 atm) ? H2O (G, 0.3 atm).
The standard molar enthalpy of vaporization is 40.7 kJ/mole. ANS in 0 decimal place
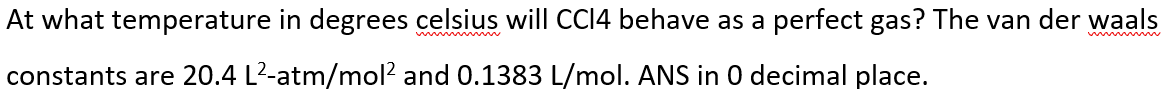
Transcribed Image Text:At what temperature in degrees celsius will CC14 behave as a perfect gas? The van der waals
constants are 20.4 L²-atm/mol2 and 0.1383 L/mol. ANS in 0 decimal place.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning