1. Calculate the pressure(P) exerted by a 0.25 mole sulfur hexafluoride in a steel vessel having a capacity o 1.25L at 343K? 2. Fermentation of glucose produce gas in the form of carbon dioxide, how many moles of carbon dioxide is produced if 0.78 L of carbon dioxide at 293K and 1.00 atm was collected during the process?
1. Calculate the pressure(P) exerted by a 0.25 mole sulfur hexafluoride in a steel vessel having a capacity o 1.25L at 343K? 2. Fermentation of glucose produce gas in the form of carbon dioxide, how many moles of carbon dioxide is produced if 0.78 L of carbon dioxide at 293K and 1.00 atm was collected during the process?
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter8: Properties Of Gases
Section8.8: The Behavior Of Real (non-ideal) Gases
Problem 8.13PSP
Related questions
Question
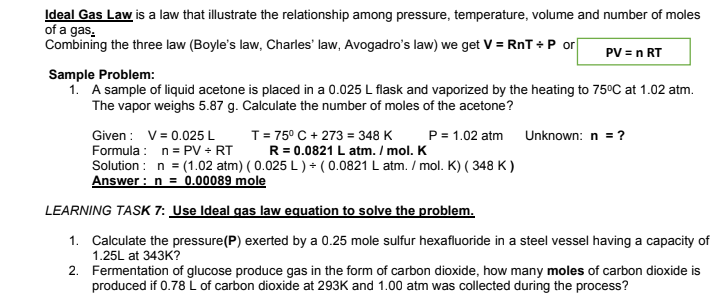
Transcribed Image Text:Ideal Gas Law is a law that illustrate the relationship among pressure, temperature, volume and number of moles
of a gas.
Combining the three law (Boyle's law, Charles' law, Avogadro's law) we get V = RnT +P or
PV = n RT
Sample Problem:
1. A sample of liquid acetone is placed in a 0.025 L flask and vaporized by the heating to 75°C at 1.02 atm.
The vapor weighs 5.87 g. Calculate the number of moles of the acetone?
Given : V= 0.025 L
Formula : n= PV + RT
Solution : n = (1.02 atm) ( 0.025 L ) + ( 0.0821 L atm. / mol. K) ( 348 K )
Answer : n = 0.00089 mole
T= 75° C + 273 = 348 K
R = 0.0821 L atm. / mol. K
P= 1.02 atm
Unknown: n = ?
LEARNING TASK 7: Use Ideal gas law equation to solve the problem.
1. Calculate the pressure(P) exerted by a 0.25 mole sulfur hexafluoride in a steel vessel having a capacity of
1.25L at 343K?
2. Fermentation of glucose produce gas in the form of carbon dioxide, how many moles of carbon dioxide is
produced if 0.78 L of carbon dioxide at 293K and 1.00 atm was collected during the process?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning