1. CN Br 2. LiAlH4 3. H₂O NH2 2a Primary amines can be prepared from nitriles by reduction with LiAlH4. The two-step sequence involves SN2 displacement of halide ion by cyanide ion followed by reduction, and yields a primary amine with one more carbon than was present in the alkyl halide. Draw curved arrows to show the movement of electrons in this step of the mechanism. Arrow-pushing Instructions Br CEN: -C=N: Br
1. CN Br 2. LiAlH4 3. H₂O NH2 2a Primary amines can be prepared from nitriles by reduction with LiAlH4. The two-step sequence involves SN2 displacement of halide ion by cyanide ion followed by reduction, and yields a primary amine with one more carbon than was present in the alkyl halide. Draw curved arrows to show the movement of electrons in this step of the mechanism. Arrow-pushing Instructions Br CEN: -C=N: Br
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter20: Dienes, Conjugated Systems, And Pericyclic Reactions
Section: Chapter Questions
Problem 20.47P
Related questions
Question
100%
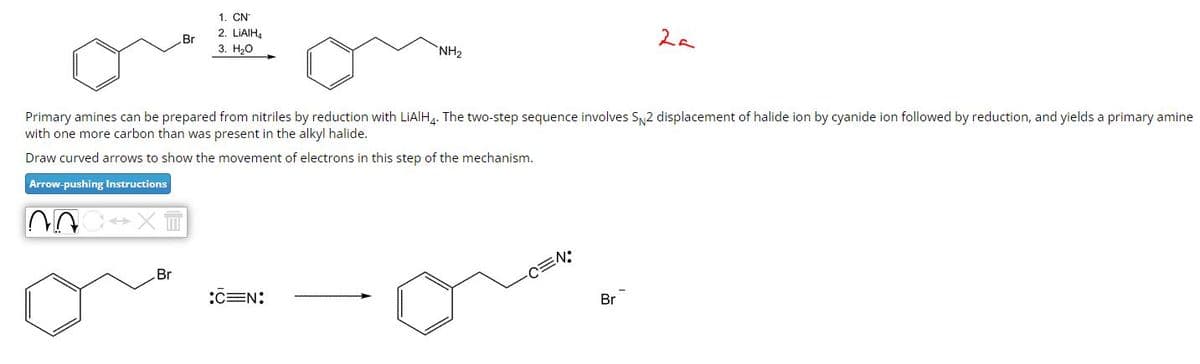
Transcribed Image Text:1. CN
Br
2. LiAlH4
3. H₂O
NH2
2a
Primary amines can be prepared from nitriles by reduction with LiAlH4. The two-step sequence involves SN2 displacement of halide ion by cyanide ion followed by reduction, and yields a primary amine
with one more carbon than was present in the alkyl halide.
Draw curved arrows to show the movement of electrons in this step of the mechanism.
Arrow-pushing Instructions
Br
CEN:
-C=N:
Br
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning