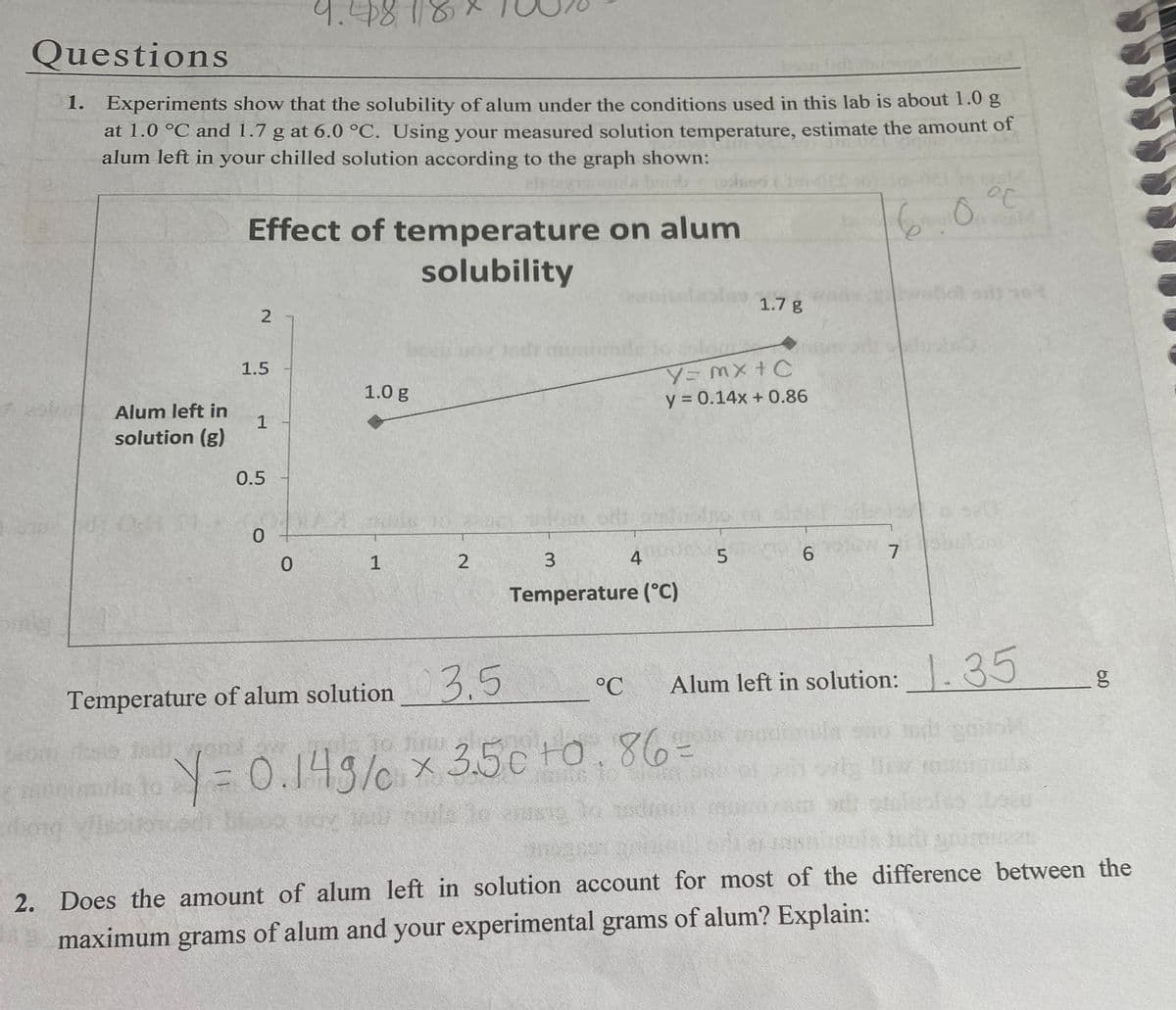
1. Experiments show that the solubility of alum under the conditions used in this lab is about 1.0 g at 1.0 °C and 1.7 g at 6.0 °C. Using your measured solution temperature, estimate the amount of alum left in your chilled solution according to the graph shown: Effect of temperature on alum solubility
1. Experiments show that the solubility of alum under the conditions used in this lab is about 1.0 g at 1.0 °C and 1.7 g at 6.0 °C. Using your measured solution temperature, estimate the amount of alum left in your chilled solution according to the graph shown: Effect of temperature on alum solubility
Chapter3: Statistical Tests With Excel
Section: Chapter Questions
Problem 7P
Related questions
Question
need help please understanding this question!!
Experiments show that the solubility of alum under conditions used in this lab is about 1.0g at 1.0 c and 1.7 at 6.0 c. Using your measured solution temperature, estimate the amount of alum left in your chilled solution according to the graph. Thank you very much!! I have no idea how to set it up and in case this shows up on an exam, I want to know how to do it!! Thank you!!!!
Transcribed Image Text:9.48 182
Questions
1. Experiments show that the solubility of alum under the conditions used in this lab is about 1.0 g
at 1.0 °C and 1.7 g at 6.0 °C. Using your measured solution temperature, estimate the amount of
alum left in your chilled solution according to the graph shown:
Effect of temperature on alum
solubility
1.7 g
Y=MX+C
y = 0.14x + 0.86
1.5
1.0 g
Alum left in
1
solution (g)
0.5
0.
1
4
Temperature (°C)
3.5
Alum left in solution: . 35
1.35
°C
Temperature of alum solution
Hoste
Y=0.143/ x3.5c to,86=
2. Does the amount of alum left in solution account for most of the difference between the
maximum grams of alum and your experimental grams of alum? Explain:
2.
2.

Transcribed Image Text:0.543
3:5"
109.18
116.27
7.09
Mass of aluminum foil used
°C
Temperature of cooled alum solution
Mass of empty 150-mL (or 250-mL) beaker
Mass of 150-mL (or 250-mL) beaker + dried alum crystals
Mass of alum obtained
%3D
116.275 109.18 7.095
6.
For the following, show your calculations:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning



Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning