1. Figure 1 gives the experimentally determined phase diagrams for the nearly ideal solution of hexane and heptane. 900 700 500 300 70°C 0.25 0.50 0.75 ZHeptane Figure 1 a) Label the regions of the phase diagram. (c) A liquid mixture is composed of 3 moles of hexane and 2 moles of heptane. If the pressure over the mixture is reduced, estimate the pressure where the first vapor formed.
1. Figure 1 gives the experimentally determined phase diagrams for the nearly ideal solution of hexane and heptane. 900 700 500 300 70°C 0.25 0.50 0.75 ZHeptane Figure 1 a) Label the regions of the phase diagram. (c) A liquid mixture is composed of 3 moles of hexane and 2 moles of heptane. If the pressure over the mixture is reduced, estimate the pressure where the first vapor formed.
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter12: Solutions
Section: Chapter Questions
Problem 12.50QE: The solubility of ethylene (C2H4) in water at 20 C and 0.300 atm pressure is 1.27 104 molal. (a)...
Related questions
Question
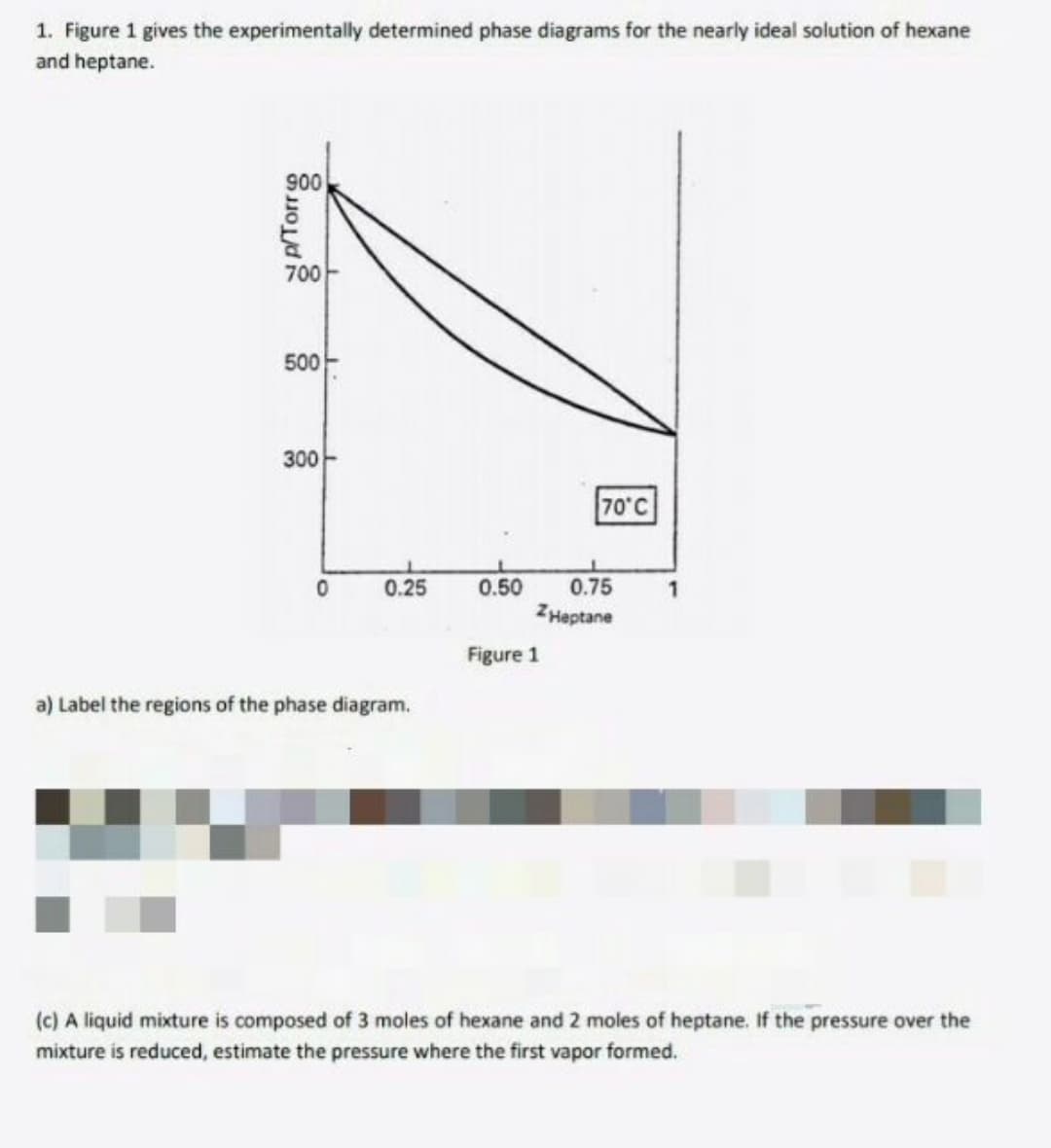
Transcribed Image Text:1. Figure 1 gives the experimentally determined phase diagrams for the nearly ideal solution of hexane
and heptane.
900
700
500
300-
70°C
0.25
0.50
0.75
ZHeptane
Figure 1
a) Label the regions of the phase diagram.
(c) A liquid mixture is composed of 3 moles of hexane and 2 moles of heptane. If the pressure over the
mixture is reduced, estimate the pressure where the first vapor formed.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning