10. Consider the following compounds when answering the questions below. Assume these compounds can be separated by TLC. A B OH C Separation of Amino Acids by Thin Layer Chromatography a. Arrange the above compounds in order of increasing Re in a TLC analysis based on their structure and how they adsorption and separation on TLC plate. (Hint: Background information document: What separates the compounds as a chromatogram develops?)
10. Consider the following compounds when answering the questions below. Assume these compounds can be separated by TLC. A B OH C Separation of Amino Acids by Thin Layer Chromatography a. Arrange the above compounds in order of increasing Re in a TLC analysis based on their structure and how they adsorption and separation on TLC plate. (Hint: Background information document: What separates the compounds as a chromatogram develops?)
Chapter89: Thin-layer Chromatography
Section: Chapter Questions
Problem 1P
Related questions
Question
Separation of Amino Acids by Thin Layer Chromatography Lab Questions. Please see photos and answer the questions relating to the images from #10. Thank you!
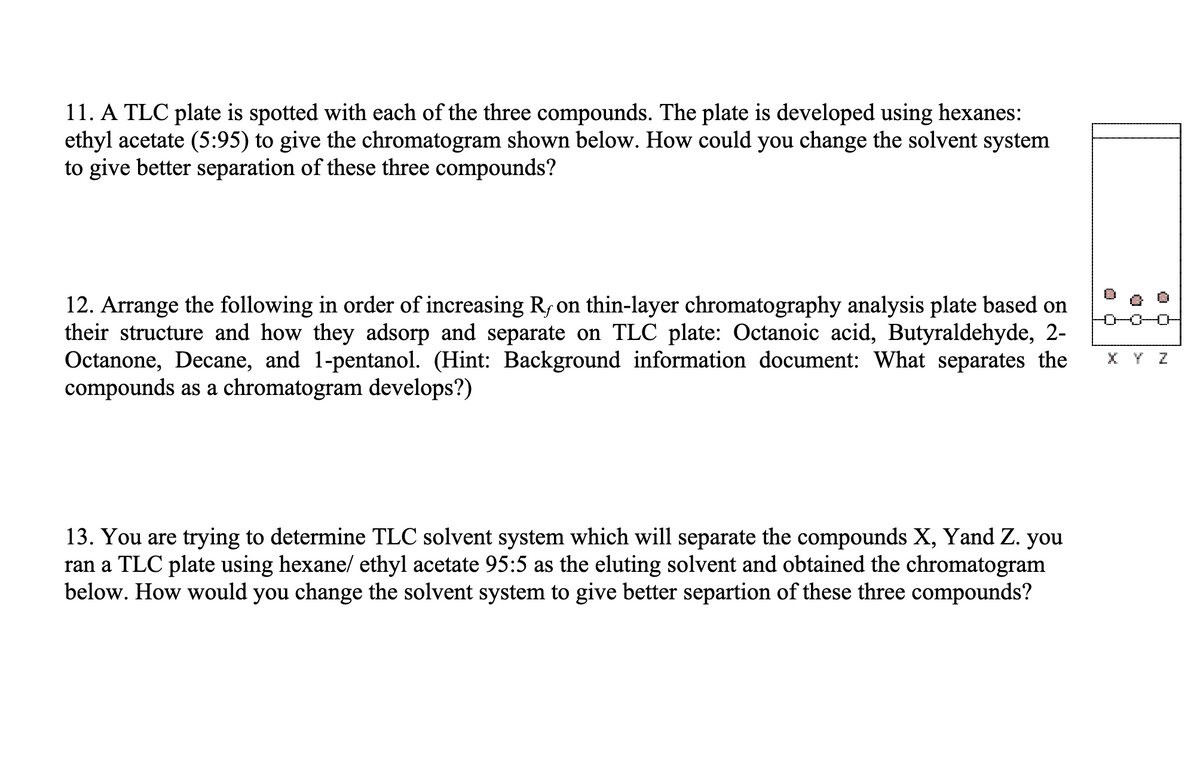
Transcribed Image Text:11. A TLC plate is spotted with each of the three compounds. The plate is developed using hexanes:
ethyl acetate (5:95) to give the chromatogram shown below. How could you change the solvent system
to give better separation of these three compounds?
12. Arrange the following in order of increasing R, on thin-layer chromatography analysis plate based on
their structure and how they adsorp and separate on TLC plate: Octanoic acid, Butyraldehyde, 2-
Octanone, Decane, and 1-pentanol. (Hint: Background information document: What separates the
compounds as a chromatogram develops?)
13. You are trying to determine TLC solvent system which will separate the compounds X, Yand Z. you
ran a TLC plate using hexane/ ethyl acetate 95:5 as the eluting solvent and obtained the chromatogram
below. How would you change the solvent system to give better separtion of these three compounds?
-0-0-0
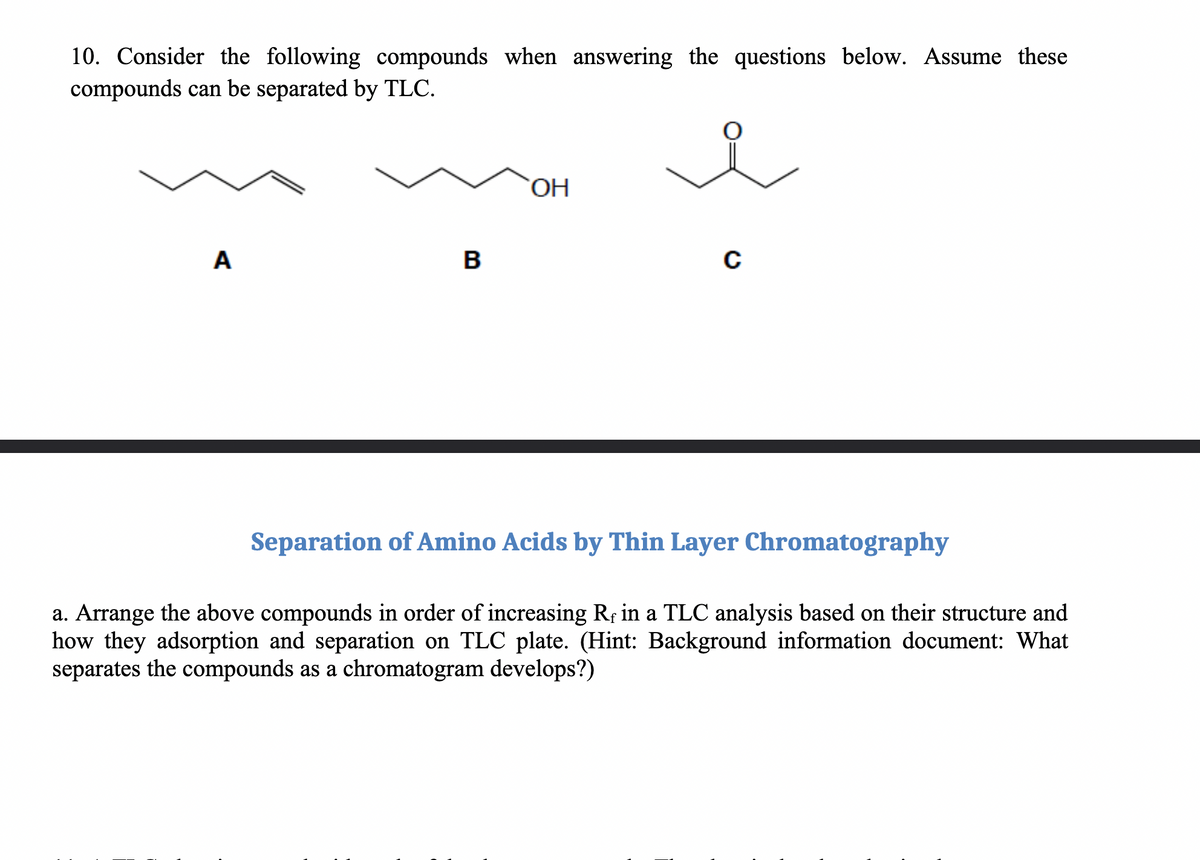
Transcribed Image Text:10. Consider the following compounds when answering the questions below. Assume these
compounds can be separated by TLC.
A
B
OH
C
Separation of Amino Acids by Thin Layer Chromatography
a. Arrange the above compounds in order of increasing Rf in a TLC analysis based on their structure and
how they adsorption and separation on TLC plate. (Hint: Background information document: What
separates the compounds as a chromatogram develops?)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning