1. Minor changes in primary structure can have pronounced effects on biological function. Tetrameric hemoglobin, ₂₂, dissociates into pairs of dimers aß to different extents depending on the protein primary structure. The standard Gibb energies of dissociation of normal adult hemoglobin A and of several mutants have been determined and are listed in the table below. Substitution in mutant Hemoglobin A, normal Richmond Kansas Georgia 1025; Asn → Thr 1028; Asn → Thr 95x; Pro → Leu AG of dissociation (kcal/mole Hb) 8.2 6.0 5.1 3.6 (a) Write an equilibrium expression for the dissociation of hemoglobin tetramer into dimers. (b) For normal hemoglobin and its three mutants (variations) calculate the equilibrium constant for their dissociation from a tetramer into dimers. (c) How do the equilibrium constants for the dissociation of the hemoglobin mutants compare to that of normal hemoglobin?
1. Minor changes in primary structure can have pronounced effects on biological function. Tetrameric hemoglobin, ₂₂, dissociates into pairs of dimers aß to different extents depending on the protein primary structure. The standard Gibb energies of dissociation of normal adult hemoglobin A and of several mutants have been determined and are listed in the table below. Substitution in mutant Hemoglobin A, normal Richmond Kansas Georgia 1025; Asn → Thr 1028; Asn → Thr 95x; Pro → Leu AG of dissociation (kcal/mole Hb) 8.2 6.0 5.1 3.6 (a) Write an equilibrium expression for the dissociation of hemoglobin tetramer into dimers. (b) For normal hemoglobin and its three mutants (variations) calculate the equilibrium constant for their dissociation from a tetramer into dimers. (c) How do the equilibrium constants for the dissociation of the hemoglobin mutants compare to that of normal hemoglobin?
Human Physiology: From Cells to Systems (MindTap Course List)
9th Edition
ISBN:9781285866932
Author:Lauralee Sherwood
Publisher:Lauralee Sherwood
Chapter11: The Blood
Section: Chapter Questions
Problem 1SQE
Related questions
Question
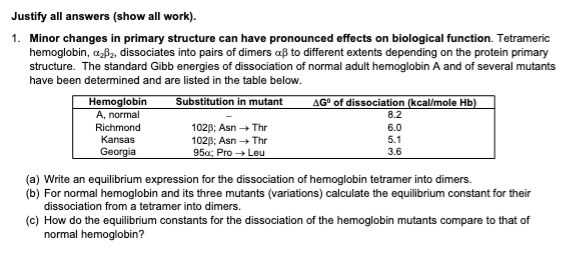
Transcribed Image Text:Justify all answers (show all work).
1. Minor changes in primary structure can have pronounced effects on biological function. Tetrameric
hemoglobin, a₂³₂, dissociates into pairs of dimers aß to different extents depending on the protein primary
structure. The standard Gibb energies of dissociation of normal adult hemoglobin A and of several mutants
have been determined and are listed in the table below.
Substitution in mutant
Hemoglobin
A, normal
Richmond
Kansas
Georgia
102B; Asn → Thr
102B; Asn → Thr
95x; Pro → Leu
AG® of dissociation (kcal/mole Hb)
8.2
6.0
65
5.1
3.6
(a) Write an equilibrium expression for the dissociation of hemoglobin tetramer into dimers.
(b) For normal hemoglobin and its three mutants (variations) calculate the equilibrium constant for their
dissociation from a tetramer into dimers.
(c) How do the equilibrium constants for the dissociation of the hemoglobin mutants compare to that of
normal hemoglobin?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Recommended textbooks for you

Human Physiology: From Cells to Systems (MindTap …
Biology
ISBN:
9781285866932
Author:
Lauralee Sherwood
Publisher:
Cengage Learning


Human Physiology: From Cells to Systems (MindTap …
Biology
ISBN:
9781285866932
Author:
Lauralee Sherwood
Publisher:
Cengage Learning
