1. Ni-cad batteries are rechargeable. The two reduction half-cells involved in the Ni-cad batteries Cd(OH)2 (s) + 2 e Cd (s) + 2OH(aq) Eº = -0.860 V are: (a) (b) NiO2 (s) + 2 H₂O (1) + 2e → Ni(OH)2 (s) + 2 OH(aq) E° = 0.490 V a. Figure out which reaction must be reversed and written as an oxidation half-reaction. Write the spontaneous overall redox reaction and calculate Eºcell. b. What must serve as the anode in this battery? What serves as the cathode? c. Write the half-reactions and overall reaction that occurs when this battery is recharged. d. After charging the above battery system for 90 min. at a current of 10 mA (0.010 A), how many grams of each of the original electrodes (anode and cathode) would be produced?
1. Ni-cad batteries are rechargeable. The two reduction half-cells involved in the Ni-cad batteries Cd(OH)2 (s) + 2 e Cd (s) + 2OH(aq) Eº = -0.860 V are: (a) (b) NiO2 (s) + 2 H₂O (1) + 2e → Ni(OH)2 (s) + 2 OH(aq) E° = 0.490 V a. Figure out which reaction must be reversed and written as an oxidation half-reaction. Write the spontaneous overall redox reaction and calculate Eºcell. b. What must serve as the anode in this battery? What serves as the cathode? c. Write the half-reactions and overall reaction that occurs when this battery is recharged. d. After charging the above battery system for 90 min. at a current of 10 mA (0.010 A), how many grams of each of the original electrodes (anode and cathode) would be produced?
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter17: Electrochemistry And Its Applications
Section17.3: Voltaic Cells
Problem 17.3PSP
Related questions
Question
See image below
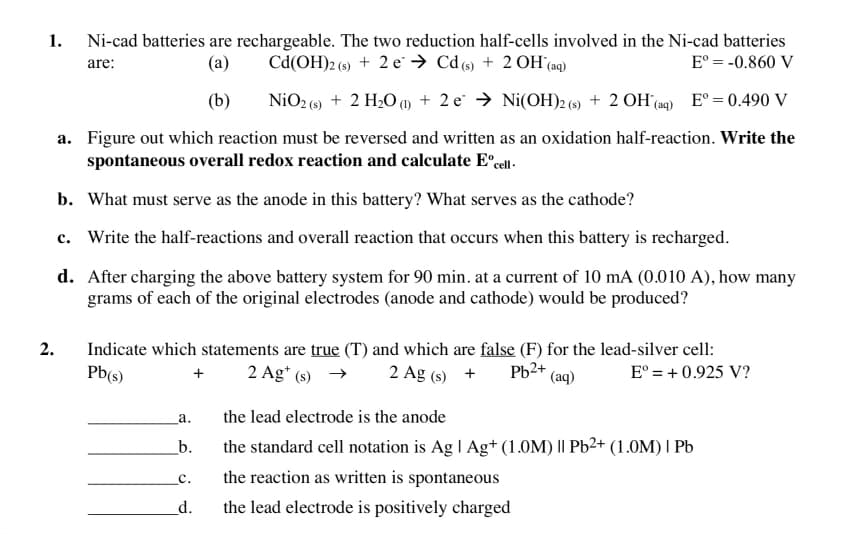
Transcribed Image Text:Ni-cad batteries are rechargeable. The two reduction half-cells involved in the Ni-cad batteries
Cd(OH)2 (s) + 2e → Cd (s) + 2OH(aq)
Eº = -0.860 V
are:
(a)
(b)
NiO2 (s) + 2 H₂O (1) + 2e → Ni(OH)2 (s) + 2OH(aq) Eº = 0.490 V
a. Figure out which reaction must be reversed and written as an oxidation half-reaction. Write the
spontaneous overall redox reaction and calculate Eºcell-
b. What must serve as the anode in this battery? What serves as the cathode?
c. Write the half-reactions and overall reaction that occurs when this battery is recharged.
d. After charging the above battery system for 90 min. at a current of 10 mA (0.010 A), how many
grams of each of the original electrodes (anode and cathode) would be produced?
1.
2.
Pb²+ (aq)
Eº = + 0.925 V?
Indicate which statements are true (T) and which are false (F) for the lead-silver cell:
Pb(s)
2 Ag* (s)
2 Ag (s) +
+
a.
b.
C.
d.
the lead electrode is the anode
the standard cell notation is Ag | Ag+ (1.0M) || Pb2+ (1.0M) | Pb
the reaction as written is spontaneous
the lead electrode is positively charged
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning