1. Requirements of Active Sites in Enzymes The active site of an enzyme usually consists of a pocket on the enzyme surface lined with amino acid side chains necessary to bind the substrate and catalyze its chemical transformation. Carboxypeptidase, which sequentially removes the carboxyl-terminal amino acid residues from its peptide substrates, consists of a síngle chain of 307 amino acids. The two essential catalytic groups in the active site are furnished by Arg145 and Glu²7º. (a) If the carboxpeptidase chain were a perfect a-helix, how far apart (in nanometers) would Arg45 and Glu²7º be? Hint: In an æ-helix, there is 1.5 Å translation of the backbone per residue (Chapter 3). (b) Explain how it is that these two amino acids, so distantly separated in the sequence, can catalyze a reaction occurring in the space of a few tenths of a nanometer. ,270
1. Requirements of Active Sites in Enzymes The active site of an enzyme usually consists of a pocket on the enzyme surface lined with amino acid side chains necessary to bind the substrate and catalyze its chemical transformation. Carboxypeptidase, which sequentially removes the carboxyl-terminal amino acid residues from its peptide substrates, consists of a síngle chain of 307 amino acids. The two essential catalytic groups in the active site are furnished by Arg145 and Glu²7º. (a) If the carboxpeptidase chain were a perfect a-helix, how far apart (in nanometers) would Arg45 and Glu²7º be? Hint: In an æ-helix, there is 1.5 Å translation of the backbone per residue (Chapter 3). (b) Explain how it is that these two amino acids, so distantly separated in the sequence, can catalyze a reaction occurring in the space of a few tenths of a nanometer. ,270
Biology: The Dynamic Science (MindTap Course List)
4th Edition
ISBN:9781305389892
Author:Peter J. Russell, Paul E. Hertz, Beverly McMillan
Publisher:Peter J. Russell, Paul E. Hertz, Beverly McMillan
Chapter6: Energy, Enzymes, And Biological Reactions
Section: Chapter Questions
Problem 8TYK: Which of the following statements about the allosteric site is true? a. The allosteric site is a...
Related questions
Question
Answer b and c
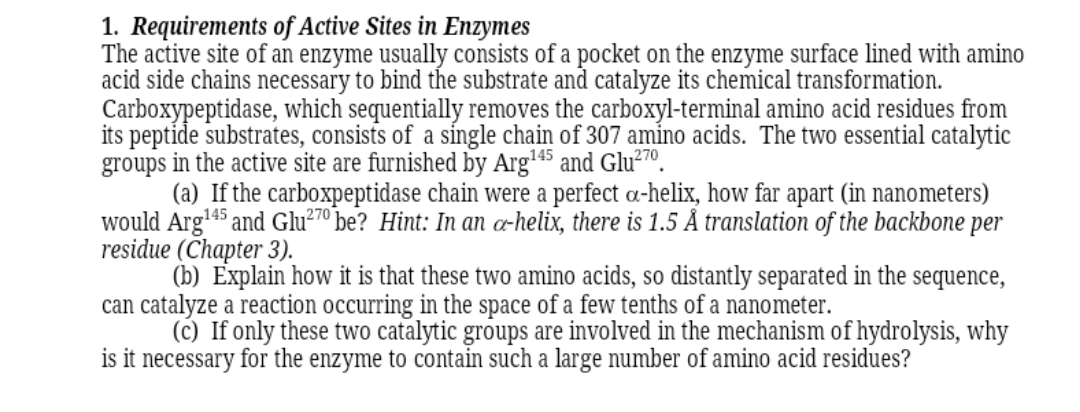
Transcribed Image Text:1. Requirements of Active Sites in Enzymes
The active site of an enzyme usually consists of a pocket on the enzyme surface lined with amino
acid side chains necessary to bind the substrate and catalyze its chemical transformation.
Carboxypeptidase, which sequentially removes the carboxyl-terminal amino acid residues from
its peptide substrates, consists of a síngle chain of 307 amino acids. The two essential catalytic
groups in the active site are furnished by Arg45 and Glu²7º.
(a) If the carboxpeptidase chain were a perfect a-helix, how far apart (in nanometers)
would Arg'45 and Glu²7º be? Hint: In an æ-helix, there is 1.5 Å translation of the backbone per
residue (Chapter 3).
(b) Explain how it is that these two amino acids, so distantly separated in the sequence,
can catalyze a reaction occurring in the space of a few tenths of a nanometer.
(c) If only these two catalytic groups are involved in the mechanism of hydrolysis, why
is it necessary for the enzyme to contain such a large number of amino acid residues?
270
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you

Biology: The Dynamic Science (MindTap Course List)
Biology
ISBN:
9781305389892
Author:
Peter J. Russell, Paul E. Hertz, Beverly McMillan
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Biology: The Dynamic Science (MindTap Course List)
Biology
ISBN:
9781305389892
Author:
Peter J. Russell, Paul E. Hertz, Beverly McMillan
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning