1. (Short Essay) In the classical model of a Hydrogen atom, we can assume that an electron (e-) orbits about a nucleus containing the neutron and proton (e+). This is known has the Bohr Model of the hydrogen atom. Derive an expression for the total energy of an electron starting with Coulomb forces of an electron orbiting in a perfect circular motion. We can note that the potential energy between charges are just like with work, - dr Lets do some research now. With Bohr's model there were flaws. Why is this model incorrect? and what is the correct model of a Hydrogen atom? Provide a citation and works that you have looked into (websites/wikipedia are OK). There is no need to write more than one page (half is sufficient). е
1. (Short Essay) In the classical model of a Hydrogen atom, we can assume that an electron (e-) orbits about a nucleus containing the neutron and proton (e+). This is known has the Bohr Model of the hydrogen atom. Derive an expression for the total energy of an electron starting with Coulomb forces of an electron orbiting in a perfect circular motion. We can note that the potential energy between charges are just like with work, - dr Lets do some research now. With Bohr's model there were flaws. Why is this model incorrect? and what is the correct model of a Hydrogen atom? Provide a citation and works that you have looked into (websites/wikipedia are OK). There is no need to write more than one page (half is sufficient). е
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter5: Quantum Mechanics And Atomic Structure
Section: Chapter Questions
Problem 55AP: The outermost electron in an alkali-metal atom is sometimes described as resembling an electron in...
Related questions
Question
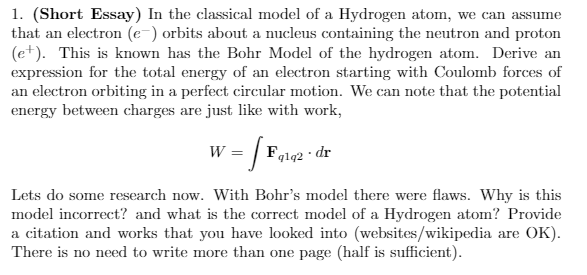
Transcribed Image Text:1. (Short Essay) In the classical model of a Hydrogen atom, we can assume
that an electron (e-) orbits about a nucleus containing the neutron and proton
(e+). This is known has the Bohr Model of the hydrogen atom. Derive an
expression for the total energy of an electron starting with Coulomb forces of
an electron orbiting in a perfect circular motion. We can note that the potential
energy between charges are just like with work,
w = |
Fglq2 · dr
W
Lets do some research now. With Bohr's model there were flaws. Why is this
model incorrect? and what is the correct model of a Hydrogen atom? Provide
a citation and works that you have looked into (websites/wikipedia are OK).
There is no need to write more than one page (half is sufficient).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 10 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning