In qualitative terms, how does Bohr's model account for the atomic spectrum of hydrogen? More than one selection may be correct. O The energy released when an electron drops from a higher to lower orbit appears as a photon with the appropriate frequency. O The energy released when an electron drops from a higher to lower orbit appears as a photon with energy equal to the energy difference between the two orbits. O Electron moves around the nucleus along orbits. O When a hydrogen atom absorbs energy, as it does when an electric discharge passes through it, the electron stays in the orbit having n = 1. O Electrons are found in orbits of arbitrary energy. O The energy released when an electron drops from a higher to lower orbit appears as a photon with an arbitrary frequency. O When a hydrogen atom absorbs energy, as it does when an electric discharge passes through it, the electron is raised from the orbit having n = 1 to a higher orbit, to n= 2 or n = 3 or even higher.
In qualitative terms, how does Bohr's model account for the atomic spectrum of hydrogen? More than one selection may be correct. O The energy released when an electron drops from a higher to lower orbit appears as a photon with the appropriate frequency. O The energy released when an electron drops from a higher to lower orbit appears as a photon with energy equal to the energy difference between the two orbits. O Electron moves around the nucleus along orbits. O When a hydrogen atom absorbs energy, as it does when an electric discharge passes through it, the electron stays in the orbit having n = 1. O Electrons are found in orbits of arbitrary energy. O The energy released when an electron drops from a higher to lower orbit appears as a photon with an arbitrary frequency. O When a hydrogen atom absorbs energy, as it does when an electric discharge passes through it, the electron is raised from the orbit having n = 1 to a higher orbit, to n= 2 or n = 3 or even higher.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter5: Electron Configurations And The Periodic Table
Section: Chapter Questions
Problem 35QRT
Related questions
Question
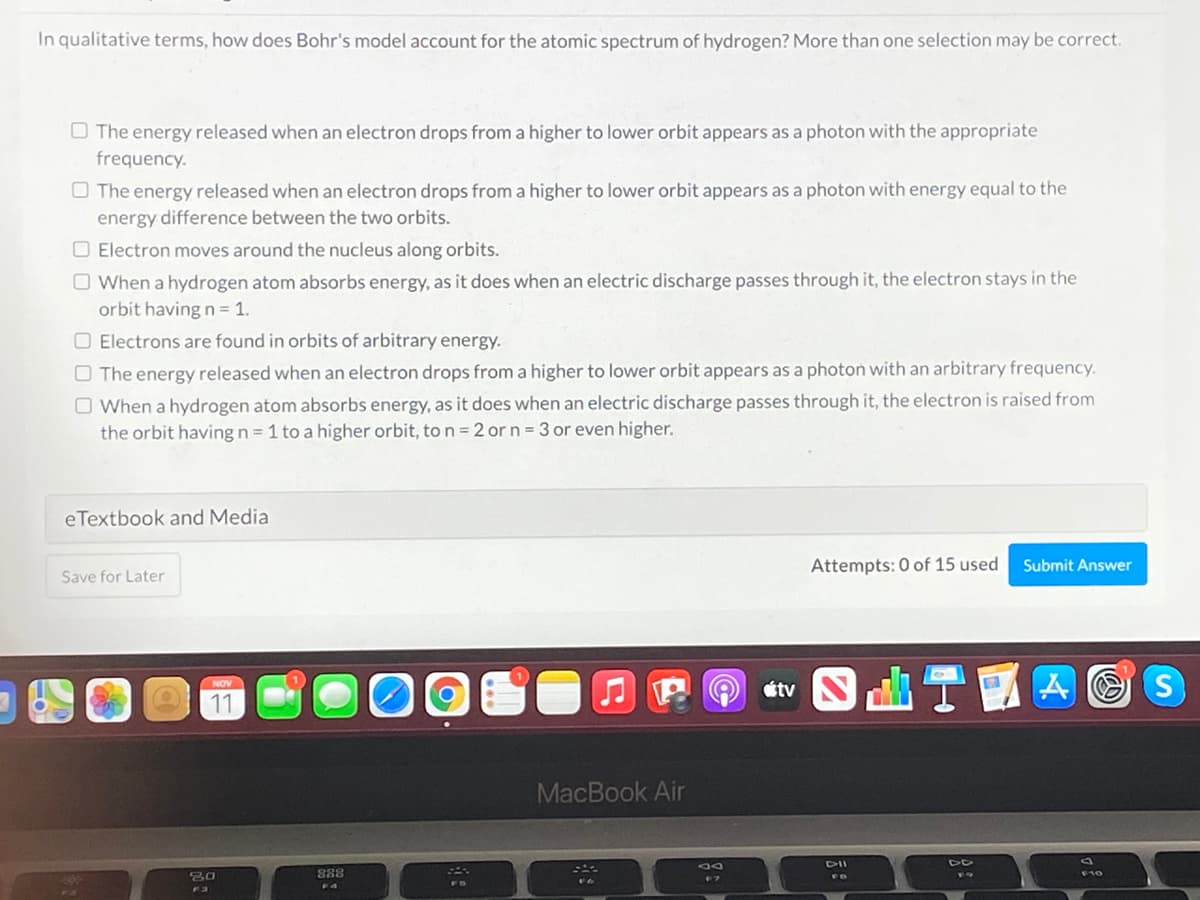
Transcribed Image Text:In qualitative terms, how does Bohr's model account for the atomic spectrum of hydrogen? More than one selection may be correct.
O The energy released when an electron drops from a higher to lower orbit appears as a photon with the appropriate
frequency.
O The energy released when an electron drops from a higher to lower orbit appears as a photon with energy equal to the
energy difference between the two orbits.
O Electron moves around the nucleus along orbits.
O When a hydrogen atom absorbs energy, as it does when an electric discharge passes through it, the electron stays in the
orbit having n = 1.
O Electrons are found in orbits of arbitrary energy.
O The energy released when an electron drops from a higher to lower orbit appears as a photon with an arbitrary frequency.
O When a hydrogen atom absorbs energy, as it does when an electric discharge passes through it, the electron is raised from
the orbit having n = 1 to a higher orbit, to n = 2 or n = 3 or even higher.
eTextbook and Media
Attempts: 0 of 15 used
Submit Answer
Save for Later
A O S
NOV
étv
MacBook Air
DII
388
F4
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning