1. The surface of silica gel has a structure shown in the diagram below : ОН OH ОН -0- Si - 0- Si- 0- Si- 0- main body of silica structure Suppose you used a plate coated with silica gel, with propanone, CH;COCH3, as the solvent for thin layer chromatography. Suppose also that the mixture you were trying to identify contained A compound, P, which could form strong hydrogen bonds. A compound, Q, which formed hydrogen bonds, but not as strongly as P. A compound, R, which was polar, relying on dispersion forces and dipole-dipole interactions for its intermolecular attractions. Describe and explain what the chromatogram would probably look like.
1. The surface of silica gel has a structure shown in the diagram below : ОН OH ОН -0- Si - 0- Si- 0- Si- 0- main body of silica structure Suppose you used a plate coated with silica gel, with propanone, CH;COCH3, as the solvent for thin layer chromatography. Suppose also that the mixture you were trying to identify contained A compound, P, which could form strong hydrogen bonds. A compound, Q, which formed hydrogen bonds, but not as strongly as P. A compound, R, which was polar, relying on dispersion forces and dipole-dipole interactions for its intermolecular attractions. Describe and explain what the chromatogram would probably look like.
Chapter31: Introduction To Analytical Separations
Section: Chapter Questions
Problem 31.18QAP
Related questions
Question
100%
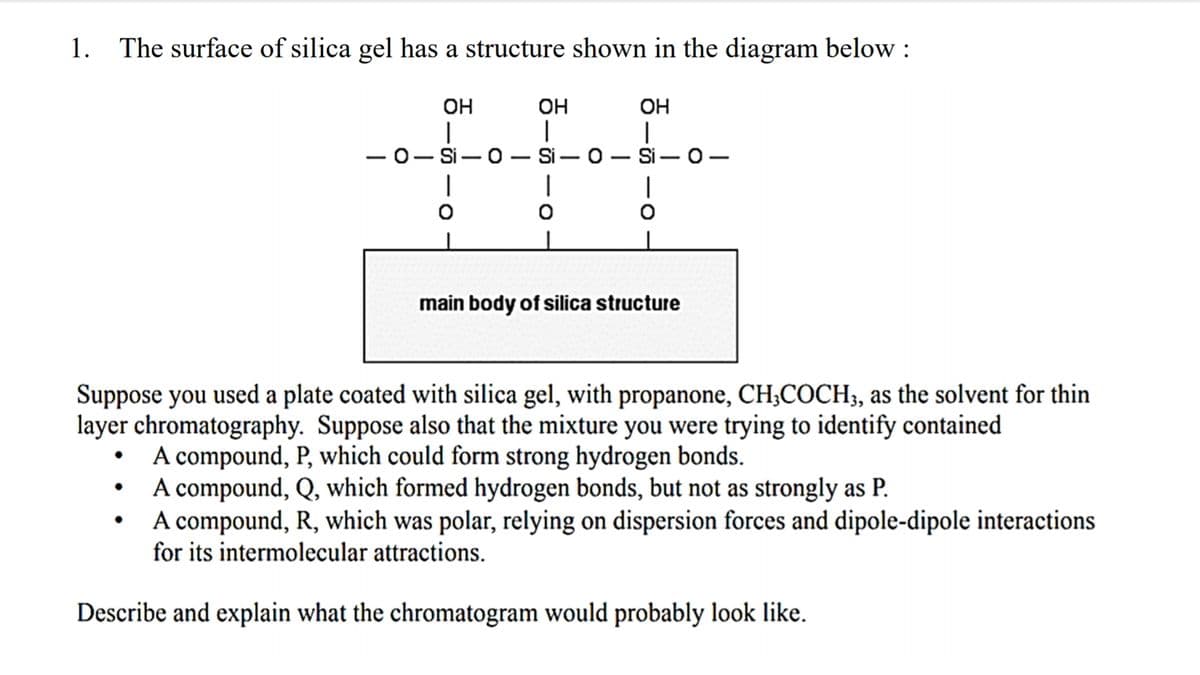
Transcribed Image Text:1.
The surface of silica gel has a structure shown in the diagram below :
OH
OH
OH
-0- Si- 0 Si
Si – 0-
main body of silica structure
Suppose you used a plate coated with silica gel, with propanone, CH;COCH;, as the solvent for thin
layer chromatography. Suppose also that the mixture you were trying to identify contained
A compound, P, which could form strong hydrogen bonds.
A compound, Q, which formed hydrogen bonds, but not as strongly as P.
A compound, R, which was polar, relying on dispersion forces and dipole-dipole interactions
for its intermolecular attractions.
Describe and explain what the chromatogram would probably look like.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole
